Questions and solutions
advertisement

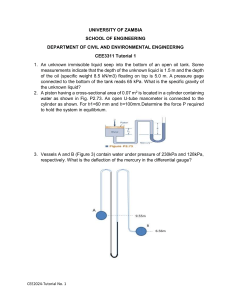
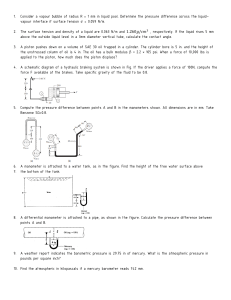
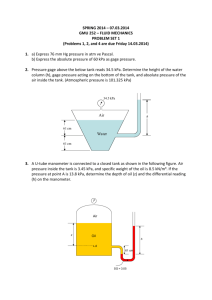
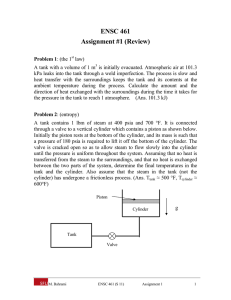
Problems Dr. Kagan ERYURUK 1) Determine the mass and the weight of the air contained in a room whose dimensions are 6 m x 6 m x 8 m. Assume the density of the air is 1.16 kg/m3. 2) Determine the atmospheric pressure at a location where the barometric reading is 750 mm Hg. Take the density of mercury to be 13,600 kg/m3. 3) A manometer containing oil (ρ = 850 kg/m3) is attached to a tank filled with air. If the oil-level difference between the two columns is 60 cm and the atmospheric pressure is 98 kPa, determine the absolute pressure of the air in the tank. 4) The top part of a water tank is divided into two compartments, as shown in figure. Now a fluid with an unknown density is poured into one side, and the water level rises a certain amount on the other side to compensate for this effect. Based on the final fluid heights shown on the figure, determine the density of the fluid added. Assume the liquid does not mix with water. 6) 5-kg rock is thrown upward with a force of 150 N at a location where the local gravitational acceleration is 9.79 m/s2. Determine the acceleration of the rock, in m/s2. 7) A can of soft drink at room temperature is put into the refrigerator so that it will cool. Would you model the can of soft drink as a closed system or as an open system? Explain. 8) Consider a system whose temperature is 18°C. Express this temperature in R, K, and °F. 9) The water in a tank is pressurized by air, and the pressure is measured by a multi-fluid manometer as shown in figure. Determine the gage pressure of air in the tank if h1 = 0.2 m, h2 = 0.3 m, and h3 = 0.46 m. Take the densities of water, oil, and mercury to be 1000 kg/m3, 850 kg/m3, and 13,600 kg/m3, respectively. 10) A gas is contained in a vertical, frictionless piston–cylinder device. The piston has a mass of 4 kg and a crosssectional area of 35 cm2. A compressed spring above the piston exerts a force of 60 N on the piston. If the atmospheric pressure is 95 kPa, determine the pressure inside the cylinder. 11) A 1.8-m3 rigid tank contains steam at 220°C. One third of the volume is in the liquid phase and the rest is in the vapor form. Determine (a) the pressure of the steam, (b) the quality of the saturated mixture, and (c) the density of the mixture. 12) A cooking pan whose inner diameter is 20 cm is filled with water and covered with a 4-kg lid. If the local atmospheric pressure is 101 kPa, determine the temperature at which the water starts boiling when it is heated. 13) A rigid vessel contains 2 kg of refrigerant-134a at 800 kPa and 120°C. Determine the volume of the vessel and the total internal energy. 14) A piston–cylinder device initially contains 50 L of liquid water at 40°C and 200 kPa. Heat is transferred to the water at constant pressure until the entire liquid is vaporized. (a) What is the mass of the water? (b) What is the final temperature? (c) Determine the total enthalpy change.