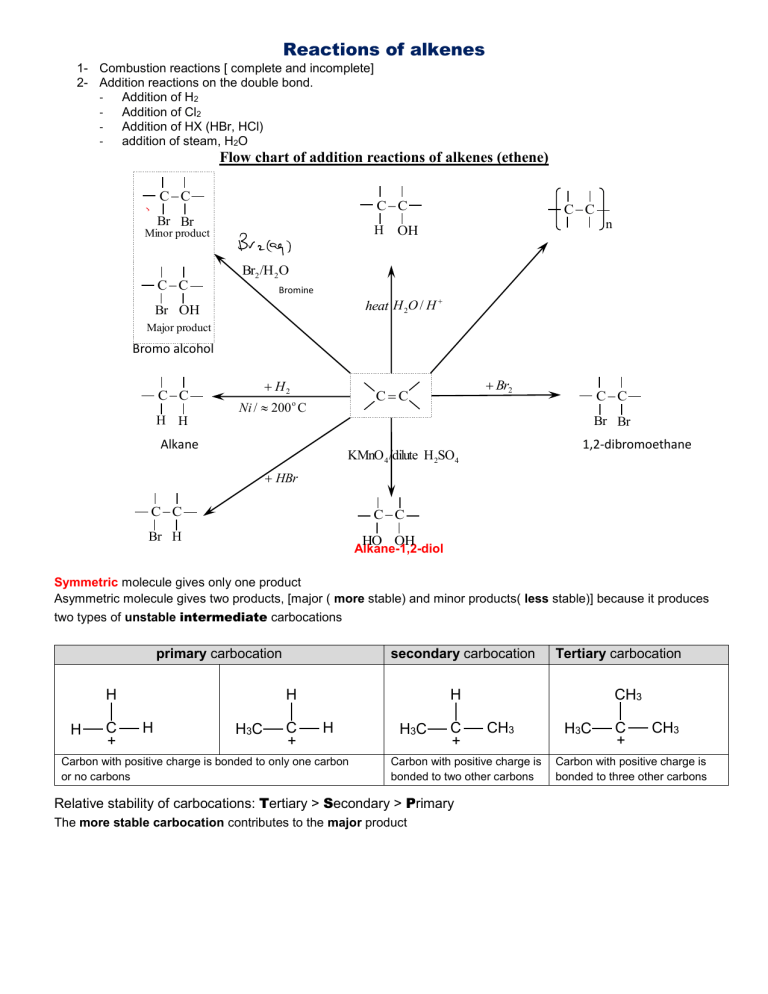
Reactions of alkenes 1- Combustion reactions [ complete and incomplete] 2- Addition reactions on the double bond. Addition of H2 Addition of Cl2 Addition of HX (HBr, HCl) addition of steam, H2O Flow chart of addition reactions of alkenes (ethene) CC CC Br Br H Minor product CC CC n OH Br2 /H 2O Bromine water Br OH heat H 2O / H Major product Bromo alcohol CC H2 Br2 CC Ni / 200 C o CC H H Br Br Alkane 1,2-dibromoethane KMnO 4 /dilute H2SO4 HBr CC CC Br H HO OH Alkane-1,2-diol Symmetric molecule gives only one product Asymmetric molecule gives two products, [major ( more stable) and minor products( less stable)] because it produces two types of unstable intermediate carbocations primary carbocation H H C + secondary carbocation H H H3C C + Tertiary carbocation H H Carbon with positive charge is bonded to only one carbon or no carbons H3C C + CH3 CH3 Carbon with positive charge is bonded to two other carbons Relative stability of carbocations: Tertiary > Secondary > Primary The more stable carbocation contributes to the major product H3C C + CH3 Carbon with positive charge is bonded to three other carbons Flow chart of addition reactions of asymmetric alkene (propene) to steam and hydrogen bromide Major product Minor product | | | | | C C C | | H | C C | OH | | C | OH H H 2O / H heat C C C HBr | | | | | | | | | | C | Br H Br H | C C C C C Major product Minor product The mechanism of Electrophilic addition of bromine to alkenes (ethene) Intermediate carbocation Electrophilic addition CC | | C C | Br Br Nucleophilic addition : Br | | C C | | Br Br Br The mechanism of Electrophilic addition of bromine to asymmetric alkene (propene) Intermediate carbocation H C | CC | Br : Br | C C Br C | Br carbocation | | C C | | | C | Br Br 1,2-dibromopropane (the only product) The mechanism of Electrophilic addition of bromine to symmetric alkene (cis or trans but-2-ene) : Br CH3 CH3 CC Br | C | Br CH3 C CH3 CH3 | | C C CH3 | | Br Br H 1,2-dibromobutane carbocation (the only product) Br The mechanism of Electrophilic addition of hydrogen bromide to Ethene Electrophilic addition CC | | C C Nucleophilic addition | H : Br | | C C | | H Br Br H The mechanism of Electrophilic addition of hydrogen bromide to asymmetric alkene (propene) | | | C C | | H C : Br C | | H Br 1-bromopropane (Minor product) Less stable | | C C | primary carbocation CC | C Br H | | Br : | | C C C | H | | | C C | | C | Br H secondary carbocation More stable 2-bromopropane (Major product) The mechanism of Electrophilic addition of HBr to symmetric alkene (cis or trans but-2-ene) carbocation CH3 CH3 CC H CH3 Br | | C | H C | CH3 CH3 C | : Br H | C CH3 | Br 2-bromobutane (the only product) Testing for alkenes (double bond) 1st test: using bromine water 1- Test : add orange bromine water 2- Result: orange turn colourless 2nd test: using acidified purple potassium managanate (VII) 1- Test : add purple potassium managanate (VII) 2- Result: decolorized / turn from purple to colourless In this reaction, the purple managanate (VII) ions are is reduced to the colourless manganese (II) ions Addition reactions of alkenes 1- addition of hydrogen type of reaction: addition because it gives only one product Reaction name: hydrogenation ( type = addition) Conditions: finely divided nickel catalyst, and 200 oC Product: alkane Ni alkane Alkene + hydrogen Cn H 2 n + Ni H 2 (word equation) (general equation) Cn H 2 n 2 H H C C H2 Ni | | | | H C C H (using structural formulae equation) H H 2- addition of halogens ( X 2 Br2 , Cl2 , I 2 ) type of reaction: addition because it gives only one product Reaction name: halogenation Conditions: room temperature and pressure Product: dihalogenoalkane ( disubstituted halogenoalkane) Alkene + halogen dihaloalkane (word equation) Cn H 2 n + Cn H 2 n Br2 (general equation) Br2 dibromoalkane Cn H 2 n + Cl2 (general equation) Cn H 2 n Cl2 dichloralkane C C Br2 brown H H | | | | H C CH Br Br 1,2-dibromoethane (Colourless) (Using structural formulae) 3- addition of hydrogen halides( HX = HBr , HCl , HI ) type of reaction: addition because it gives only one product Reaction name: hydrohalogenation Conditions: room temperature and pressure /very rapid Product: monohalogenoalkane ( monosubstituted halogenoalkane) Alkene + hydrogen halide Cn H 2 n + HBr monohaloalkane (word equation) (general equation) C n H 2 n1 Br monobromoalkane Cn H 2 n + HCl (general equation) Cn H 2 n1Cl Monochloroalkane Remark: the addition of HX to asymmetric alkene such as propene, produces two types of carbocations, generally primary and secondary. The product from secondary carbocation more stable and called major product because secondary carbocation is relatively more stable than the primary carbocation. H H H C C C asymmetric alkene HBr | | | | | | H - C C C H H Br H 2-bromoalkane Major product because the produced intermediate product, secondary carbocation, is more stable than primary carbocation. H H H | | | | | | H - C C C H minor product because the produced intermediate H H Br product, primary carbocation, is less stable than secondary carbocation 1-bromopropane Comparison between atom economy and percentage yield of producing monosubstituted alkane an alkane by free radical substitution and from alkenes by electrophilic addition higher atom economy from addition reaction of an alkene because in alkenes the monosubstituted alkane is the only one product, no by-products such as HBr, HCl,.. Higher yield from alkenes because no di-, tri- etc 4- addition of water ( H 2O ) type of reaction: addition because it gives only one product Reaction name: hydration Conditions/reagents: sulphuric acid and heat Product: alcohol Alkene + water (steam) Cn H 2 n + H OH alcohol (word equation) (general equation) Cn H 2 n1OH alcohol C C C H OH H H H | | | | | | H - C C C H Major product H OH H Asymmetric alkene Propan-2-ol H H H | | | | | | H - C C C H minor product H H OH Propan-1-ol 5- addition of bromine water (orange) ( H2O,Br2 mixture ) used as a test for alkenes / double bond Conditions/reagents: H 2O, Br2 mixture Products: 2-bomoalkanol (major) and 1,2-dibromoalakane(minor) C C Br2 H 2O OH Br | | | | H H H C CH Major product 2-bromoethanol Br Br | | H C CH | | H H 1,2-dibromoethane minor product 6- Reaction of alkenes with acidified potassium manganate (VII) used as a test for alkenes / double bond Conditions/reagents: purple KMnO4 and dilute sulphuric acid Product: alkane-1,2-diol or alkane-2,3-diol [ depending on the position of the C=C] H OH OH H KMnO 4 /dilute H2SO4 C C H | | H C C H H | | H H Ethane- 1,2- diol 7- Polymerization Polymer: a long chain molecule made up by joining small units (molecules) together. These small molecules that are joined together called monomers Addition polymerisation Definition: joining small unsaturated identical molecules (monomers) to form large saturated molecule (polymer) The double bonds break and all monomers join together forming the polymer. X Y | | 7 C C | | Z W X Y | | C C | | Z W Monomer one repeat unit - X Y | | C C | | Z W X Y | | C C | | Z W X Y | | C C | | Z W X Y X Y | | | | C C C C | | | | Z W Z W X Y | | C C | | Z W part of the polymer showing 7 repeat units one repeat unit X Y | | C C | | Z W General equation of addition polymerisation : X Y | | 100o C n C C 100atm, traces ofair | | Z W X Y | | C C | | Z W n Where X, Y, Z and W are any atoms or groups of atoms Features of addition polymerisation: - The monomer contains CC The polymer is the only product The repeat unit is the same as the monomer; except that the repeat unit has single bonds only, while the monomer has one C C . It is called addition polymerisation because the polymer is the only product and the monomer contains a double bond Empirical formula of all poly(alkenes) is CH2 because their smallest whole number ratio of C:H is 1:2 Big note: - all addition polymers are synthetic - synthetic polymers are non-biodegradable nonbiodegradable = cannot be broken down by bacteria and microorganisms Hint: Whenever you are given an unsaturated monomer, it is better to represent it in this form | | CC | | A- Poly(alkenes) n alkene poly(alken e) monomer 1- polymer Poly(ethene) - monomer: ethene gas - Product: poly(ethene) Conditions are not required Equation of polymerisation H | H | 200o C 100atm, traces of air n C C | | H H ethene monomer gas H | repeat unit: H | H | | H | H CC n poly(ethene) polymer solid H | H | | H | H CC CC | H H | | H H | H H | | H | | H | | H H | H CC CC part of the polymer showing 3 repeat units Uses of ethene: To manufacture 1.poly(ethene), 2.ethanol, 3.dry clean liquid (dichloroethane) and as 4.fuel 2- Poly(propene) H | CH3 | nC C | | H H propene gas solid monomer 200o C H | CH3 | | H | H CC 100atm, traces of air poly(propene) One repeat unit n H | CH3 H | | | H | H CC polymer CH3 H | | CC | H | H CH3 | CC | H | H part of the polymer showing 3 repeat units 3- Poly(but-1-ene) o 100 C n CH3 CH 2 CH CH 2 100 atm H H | | C C | | C2H5 H n H H | | C C | | C2H5 H H H | | C C | | C2H5 H 4- Poly(but-2-ene): o 100 C n CH3 CH CH CH3 100 atm H | C | CH3 H | C | CH3 n H | C | CH3 or H | C | CH3 H | C | CH3 H | C | CH3 Uses of poly (alkenes) To manufacture plastic bags, bins, buckets, bowls, insulators and reagent bottles B- Other addition Polymers (a) Poly(phenylethene): polystyrene n C6 H 5 CH CH 2 100 atm phenylethene H H | | C C | | C6H5 H H H | | C C | | C6H5 H H H | | C or C | | C6H5 H n o 100 C 2 repeat units Used in making disposable cups, plates, etc… H H | | C C | | C6H5 H One repeat unit (b) Poly(acrylonitrile): H | C | CN o 100 C n CN CH CH2 100 atm acrylonitrile H H | | C C | | CN H H | or C | H n H | C | CN H | C | H H | C | CN H | C | H 3 repeat unit Used in making beds, furniture and clothes H | C | CN One repeat unit (c) Teflon F F | | n CF2 CF2 C C 100 atm | | 1,1,2,2-tetrafluoro ethane n F F Teflon F F F F o 100 C or | | | | | | | | C C C C F F F F Used as nonstick layer in frying pans [ has very high melting point] (d) Poly(vinyl chloride) Monomer : Vinyl chloride CH2 CHCl o n CH2 CHCl 100 C 100 atm H | C | H H | C | Cl n or Uses: To manufacture plastic pipes, water bottles, insulators and table covers H | C | Cl H | C | H H | C | Cl H | C | H H | C | H Using skeletal formula polymerisation of propene One repeat unit n n propene Poly(propene) two repeat units polymerisation of cis - butene One repeat unit n n Cis-butene Poly(butene) two repeat units polymerisation of 1,2-dimethyl cyclohexene One repeat unit n n Cis-butene Poly(butene) two repeat units Comparison between the properties of the monomer and the polymer Monomer “Alkene” property - physical - - chemical - monomer Polymer Uses of the polymer Physical properties gas because it is simple molecule; has weak intermolecular forces that are broken by only little energy. reactive because it has C=C, it undergoes addition reactions Ethene Poly(ethene) Polymer “poly(alkene)” - solid because it has strong intermolecular forces as it is large molecule. - unreactive because it has only strong single bonds Propene Poly(propene) Vinyl chloride Poly(vinyl chloride) PVC Packaging, Films, ropes that Water pipes and utensils don’t rot storages Thermoplastics: Can be resoftened and molded into new shapes when warmed Tetrafluoroethene Teflon In nonstick frying items Very high melting point Low –density poly (ethene)= LDPE: has highly branched chain with fairly low melting point. It is soft and malleable. High –density poly (ethene)= HDPE: has few branched chain with low melting point. It is more rigid than LDPE and melts at higher temperature. Both types soften as it is warmed the raw material for all poly(alkenes) is crude oil because alkenes are obtained by cracking of alkanes from crude oil. Remember that all addition polymers are nonbiodegradable Environmental problems associated with polymers industry: Not easy to dispose Non-biodegradable: are not broken down by microorganisms as bacteria and fungi Burning plastics produces a variety of toxic gases, like hydrogen chloride from PVC and hydrogen cyanide which are damaging both to human health and to other living organisms. Burning plastics also increase the carbon footprint made from valuable finite resources, fossil fuels large amount of energy is used in both manufacturing the polymers and the products made from them. problems with disosal of waste from polymers - Increases landfill sites ( as they are non-biodegradable – take long time to decay) - Harmful to marine life / harmful to wildlife - when they are burned a variety of toxic gases may be produced, like hydrogen chloride from PVC and hydrogen cyanide which are damaging both to human health and to other living organisms such as marine life. Solutions to the problems of waste from polymers - Biodegradable plastics are now being developed from lactic acid (2-hydroxypropanoic acid), and 3-hydroxypropanoic acid which forms a polymer known as biopol. - Soluble polymers such as poly(ethenol), are used to make liquitabs (detergents in water soluble packets) and laundry bags for use in hospitals, to avoid handling of contaminated laundry. - Recycling thermoplastics ( plastics that soften on heating and thus can be reshaped into new items) Advantages of Recycling 1reduces disposal demand, 2 reduces emission of gases such as CO and SO and 2 2 3-both saves energy and fossil fuel finite resources Using renewable energy sources such as solar and wind energy in the manufacture of polymers Use fabric or paper bags instead of plastic ones in supermarkets Recover some of the energy used in producing the polymers by burning plastics and other polymers and use the energy released to generate electricity Incineration = disposal of polymers by burning - What is incineration? Ans: disposal of polymers by burning Adverse effect of Incineration Incineration of any chlorinated polymer releases HCl and other chlorinated compounds which are corrosive/toxic /cause acid rain How to reduce the adverse effect of incineration of polymers Ans: pass the gasses through a base such as aqueous NH3, watered CaCO3, CaO,…..), it reacts with HCl(g) and neutralises it. Solved important questions 1. Explain why incineration is not a suitable method for disposal of poly(chloroethene) Ans: incineration produces HCl (1) which is corrosive/toxic /cause acid rain (1) allow chlorinated molecules IGNORE carbon dioxide and its consequences as it is product of combustion of any fuel. don’t allow chlorine [because Chlorine is not a product of incineration] 2. Domestic waste is also disposed of by incineration. Outline one advantage of this method (incineration). Ans: reduction in bulk/ useful energy can be generated Objective be able to discuss the reasons for developing alternative fuels in terms of sustainability and reducing emissions, including the emission of CO2 and its relationship to climate change. Objective: be able to apply the concept of carbon neutrality to different fuels, such as petrol, bioethanol and hydrogen Terms to know: Nonrenewable fuel, renewable fuel, Biofuel, Anthropogenic, Carbon neutrality, Nonrenewable fuel: used up faster than it is formed; it is a finite resource/it takes millions of years to produce/ it will ‘run out’ renewable fuel: doesn’t ‘run out’ / from nonfinite resources Biofuel = fuel produced from plants such as bioethanol which is ethanol produced by fermentation of sugar from plants Anthropogenic = climate change is man-made change. Examples on anthropogenic release of CO2 and SO2 gases from combustion of fossil fuels and NO2 from reaction between N2 and O2 from air inside car’s engines at high temperature. Carbon neutrality = when the CO2 released on combustion is equal to the CO2 absorbed on formation of the fuel/by plant in photosynthesis biofuels are unlikely to be completely carbon neutral BECAUSE CO2 is likely to be released from combustion of fossil fuel during transport and production of biofuel A biofuel is cosidered carbon neutral if the amount of carbon dioxide absorbed by plants in photosynthesis (when plants grow up) equals the amount of carbon dioxide released when it is burnt. hydrogen is unlikely to be completely carbon neutral BECAUSE CO2 is likely to be released from combustion of fossil fuel during transport and production of hydrogen by electrolysis or cracking Measures by which the chemical industry could reduce its carbon footprint. Use biofuels (as they have very small carbon footprint) - Use catalysts to reduce consumption of energy from fossil fuels - Use nuclear power energy - Use renewable energy sources such as wind power, solar power and fuel cells - neutralising with scrubbers such absorbing with alkali/ limestone in water pass acidic gases such as HCl through aqueous ammonia/ NaO, CaCO3,… - harmful consequence to a person of increased exposure to ultraviolet radiation are - skin cancer - affects eye retina a. Objective know that pollutants, including carbon monoxide, oxides of nitrogen and sulfur, carbon unburned hydrocarbons, are emitted during the combustion of alkane fuels particulates and 1. How and why is SO2 gas formed during combustion of fossil fuels? Crude oil contains small amount of sulfur; 2. on burning alkanes, sulfur in fuel burns forming SO2 gas. SO2 released to the atmosphere combines with water vapour and forms sulfuric acid that falls as acid rain Consequences of acid rain damages limestone building, harms aquatic life, damages forests, and …. - carbon particulates ( black soot) from unburned carbon are dangerous to health - unburned hydrocarbons due to fuel - oxygen unbalance are harmful to health. How is NO gas formed? Ans: Under high temperature inside vehicles engines, N2 and O2 in air react forming oxides of nitrogen Equation of the reaction: N2(g) + O2(g) → 2NO(g) Then NO reacts with more O2 in air forming NO2. 2NO(g) + O2(g) → 2NO2(g) How is the amount of NO and CO released to the atmosphere decreased? Ans: By passing them through the catalytic converter, it changes the dangerous gases, NO and CO into non harmful gas N2 and CO2. It also changes unburned hydrocarbons ( fuel) into CO2 and H2O The reaction that takes place inside the catalytic converter 2NO(g) + 2CO(g) → N2(g) + CO2(g) Catalytic converter However, CO2 is a greenhouse gas that contributes to global warming when its concentration increases in the atmosphere. Answered questions 1. When does complete combustion occur? Ans: enough oxygen/ O2. 2. When are the products of incomplete combustion? Ans: carbon dioxide and water 3. Write the equation for complete combustion of ethane. State symbols are not required. Ans: C2H6 + 3½O2 → 2CO2 + 3H2O Similar equations are written the same way – must be balanced 4. State and explain the main environmental problem arising from the complete combustion of alkane fuels. Ans: - Environmental problem: Global warming (1) 1. Explanation: Due to increase in concentration of the greenhouse gas CO2 in the atmosphere CO2 traps IR rays radiated from the earth surface (1) Ignore references to the effects of climate change 5. When does incomplete combustion occur? Ans: Insufficient oxygen/ not enough O2. 6. When are the products of incomplete combustion? Ans: carbon, carbon monoxide, carbon dioxide and water 7. An incomplete combustion of methane, CH4, results in the formation of carbon monoxide and water only. Write the equation for this reaction. State symbols are not required. Ans: CH4 + 1½O2 → CO + 2H2O Similar equations are written the same way – must be balanced 8. State two problems that result from the incomplete combustion of alkane fuels. Ans: - CO is toxic / poisonous (1) Unburned hydrocarbons react to form compounds which are toxic / harmful (1) Carbon soot ( particulates) cause global dimming 9. Explain why carbon monoxide released from incomplete combustion of fossil fuels is toxic. Ans: - the released CO binds with hemoglobin and prevents oxygen from reaching body cells which leads to death. (causes asphyxia)CO is toxic / poisonous (1) 10. Explain the environmental problem and the adverse effect on health associated with carbon soot ( particulates) from incomplete combustion of fossil fuels. Ans: - Carbon soot ( particulates) cause global dimming Carbon soot cause respiratory problems especially in people with asthma 11. Give TWO factors which have to be considered when deciding which material, PVC or metal, contributes to more sustainable uses of resources in the long term. Ans: - PVC will last longer than iron due to lack of corrosion (1) PVC comes from oil which is non-renewable (1) PVC and metals come from non-renewable sources (1) Alkenes have geometric isomerism becuase C=C prevents rotation each C in the C=C double bond must be bonded to two different atoms or groups