Science 7 Test: Scientific Method, Solutions, Mixtures
advertisement

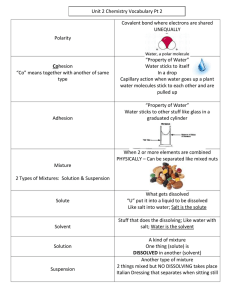
Science 7 First Quarter Long Test 1. Why is the Scientific Method an important process in doing experiments? A. It ensures that the people doing the experiments are scientific. B. It takes more work but it's worth it. C. It ensures that the results can be trusted and repeated. D. It helps the experiment to take longer and be better. 2. The first step in the scientific method is _____________________. A. Making observations C. Conducting experiments B. Analyzing data D. Defining the Problem/asking the question 3. When we make an educated guess, we are forming_____________________. A. Data B. Variables C. a Hypothesis D. a Factor 4. Our recorded observations are called_____________________. A. Data B. Hypothesis C. Controlled Variable D. Experiments 5. At the end of the scientific method, we must state a _____________________. A. Purpose B. Hypothesis C. Question D. Conclusion 6. A solution in which no more solid will dissolve is called _____________________. A. Insoluble B. Saturated C. Solubility D. Soluble 7. Solution is a homogenous mixture. Which of the following is an example of a solution? A. Mud in a water B. Ice cream C. Mango shake D. Vinegar 8. What is the most comment solvent in everyday life? A. Carbon containing chemicals B. Ethanol C. Water D. All of the above 9. Which of the following terms refers to a solution that contains the maximum quantity of solute at a given temperature? A. supersaturated solution C. saturated solution B. aqueous solution D. unsaturated solution 10. There are solution which do not appear in nature instead they are made by man. Which is an example of manufactured solution? A. Seawater B. Blood C. Cologne D. Air 11. _____________________ is a variable that is being tested or manipulated. A. Dependent B. Independent C. Controlled D. Constant 12. When a scientist shares her finding with other scientists, she is _____________________. A. Experimenting C. Analyzing Data B. Making hypothesis D. Communicating results 13. Which of the following is NOT a compound? A. HCl B. NaCl C. Cl D. CO2 14. What cannot be broken down into other substances? A. Compound B. Solids C. Mixture D. Element 15. What is made up two or more elements that are chemically combined? A. Compound B. Solids C. Mixture D. Element 16. Milk and oatmeal is an example of _____________________. A. Compound B. Element C. Heterogeneous D. Homogeneous 17. _____________________ is a form of matter that has constant chemical composition and characteristic properties. A. Pure Substance B. Mixture C. Heterogeneous D. Homogeneous 18. Describe a solute. A. part of solution present in largest amount C. gets dissolved B. water D. dissolves other substance 19. Solution where more solute can still be dissolved at the given temperature. A. Saturated B. Mixture C. Unsaturated D. Homogeneous 20. A solution has two parts. These two parts are called _____________________. A. solvent & solvation C. solvent & water B. Solvent & solute D. Solute & solvation 21. What is a solution that has the maximum amount of solute dissolved at a specific temperature and pressure? A. Saturated B. Mixture C. Unsaturated D. Undefined 22. When solute dissolves into a solvent it is called a _____________________. A. Mixture B. Element C. Solution D. Substance 23. _____________________ is the process of dissolved solute returning to the solid state A. Solution Equilibrium B. Recrystallization C. Saturated D. Unsaturated 24. Water is an example of _____________________. A. Solute B. Solvent C. Element D. Mixture 25. Milk is an example of _____________________. A. Solute B. Solvent C. Element D. Mixture 26. In a sugar solution, which component of the solution is a solute? A. Water B. Sugar C. Salt D. Milk powder 27. 5.45 g of NaCl is added to 100 mL of water. What is its percentage by mass? A. 2.23% B. 5.17% C. 5.23% D. 4.23% 28. Which statement describes the solute? A. It is the solid formed in solution B. It is the liquid part of the solution C. It is the component of a solution in smaller amount D. It is the component of a solution in bigger amount 29. Sophia wants to make a juice after lunch. What makes the powdered juice dissolve faster? A. Add water C. Pour sugar B. Wait until it will be mixed D. Stir very well 30. _________________ is a solution with commonly known concentrations. A. Solute B. Solvent C. Stock D. Concentration 31. Jay combines water, sugar, and lemon juice to form lemonade. What type of substance was made? A. Compound B. Pure Substance C. Solution D. Element 32. Which of the following is an element? A. Sugar B. Water C. Salt D. Oxygen 33. Carbon Dioxide is a _____________________. A. Solution B. Compound C. Mixture D. Element 34. What of the following element with symbol Fe? A. Boron B. Francium C. Fluorine D. Iron 35. Humans absorb oxygen from the air they breathe in and exhale carbon dioxide gas, CO2. Carbon dioxide gas is _____________________. A. an element made of carbon and oxygen atoms B. a compound of carbon and oxygen atoms C. a solution made of carbon and oxygen atoms D. a mixture of carbon and oxygen atoms 36. What are the two types of pure substances? A. acid and base C. metals and nonmetals B. element and compound D. compound and mixture 37. You are asked to make a homogeneous mixture. Which of the following set-ups will you choose? A. Oil and water B. Vinegar and water C. Rice and water D. Sand and gravel 38. A mixture that does not look the same throughout is a _____________________. A. Compound B. Element C. Heterogeneous D. Homogeneous 39. Which of the following is a pure substance? A. Bread B. table salt C. garden soil D. sea water 40. Which of the following is a way in which elements and compounds are similar? A. Elements and compounds are both pure substances. B. Elements and compounds are both listed on the periodic table. C. Elements and compounds are both made up of different kinds of atoms. D. Elements and compounds can both be broken down by physical changes. Prepared by: Checked by: CRISTINE JOY S. HIRANG MARIVI D. LADINES Subject Teacher Teacher-in-Charge 4. How does temperature affect solubility? a. Solubility is not affected by temperature b. Solubility decreases with an increase in temperature c. Solubility increases with an increase in temperature. d. Solubility increases with a decrease in temperature. 5. The rate of solution is a measure of how fast a substance dissolves. What are the factors that determine the rate of solution? a. Stirring b. Temperature c. Amount solute already dissolved d. Time 6. What are the main factors that affect solubility? a. Temperature c. Nature of solute and solvent b. Pressure d. All of the above 7. As the temperature of a liquid solvent increases, the amount of solute that can dissolve in it a. decreases by one degree Celsius for every milliliter of solvent b. increases c. decrease d. remains constant 13. To determine whether the mixture is a solution or not the following are its characteristics, EXCEPT; a. Clear c. Homogeneous b. Transparent d. Can be filtered 14. How will you describe a mixture out of salt and oil? a. The oil can dissolved into the salt. b. The oil can only dissolved a little bit of a salt. c. It becomes a homogenous solution, the salt is miscible into the oil. d. It becomes a heterogeneous mixture, the salt is immiscible into the oil.