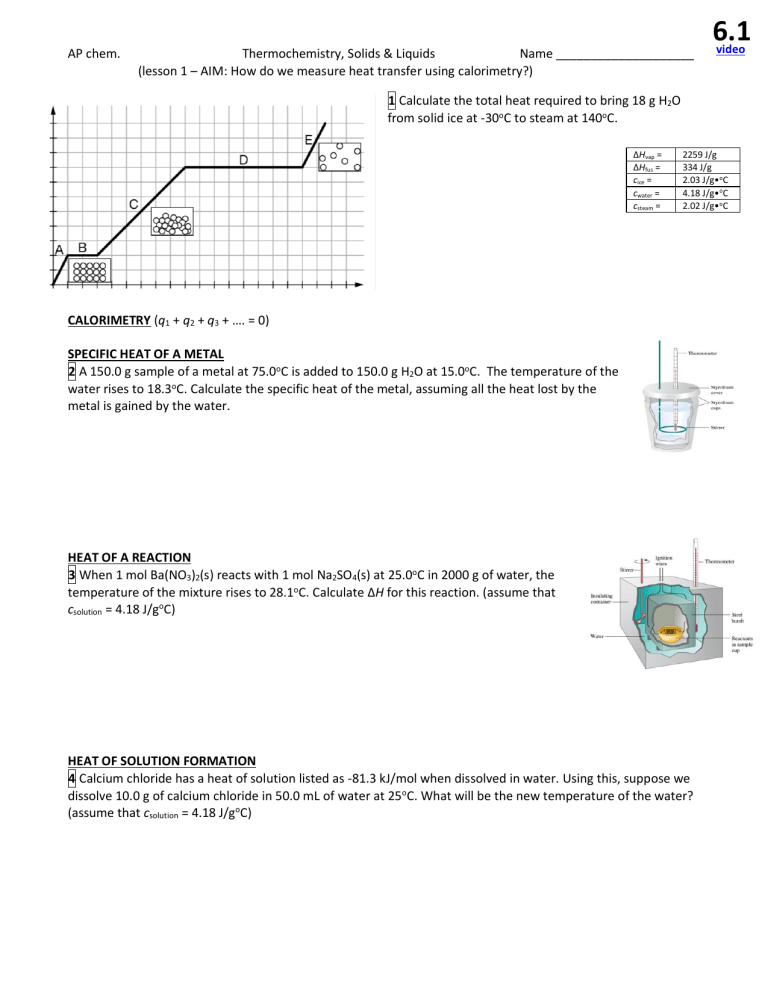
AP chem. Thermochemistry, Solids & Liquids Name ____________________ (lesson 1 AIM: How do we measure heat transfer using calorimetry?) 6.1 video 1 Calculate the total heat required to bring 18 g H2O from solid ice at -30oC to steam at 140oC. Hvap = Hfus = cice = cwater = csteam = 2259 J/g 334 J/g CALORIMETRY (q1 + q2 + q3 SPECIFIC HEAT OF A METAL 2 A 150.0 g sample of a metal at 75.0oC is added to 150.0 g H2O at 15.0oC. The temperature of the water rises to 18.3oC. Calculate the specific heat of the metal, assuming all the heat lost by the metal is gained by the water. HEAT OF A REACTION 3 When 1 mol Ba(NO3)2(s) reacts with 1 mol Na2SO4(s) at 25.0oC in 2000 g of water, the temperature of the mixture rises to 28.1oC. Calculate H for this reaction. (assume that csolution = 4.18 J/goC) HEAT OF SOLUTION FORMATION 4 Calcium chloride has a heat of solution listed as -81.3 kJ/mol when dissolved in water. Using this, suppose we dissolve 10.0 g of calcium chloride in 50.0 mL of water at 25oC. What will be the new temperature of the water? (assume that csolution = 4.18 J/goC) o C C oC o AP chem. Thermochemistry, Solids & Liquids Name ____________________ PROBLEM SET 6.1 5 40 g ice at 0oC is mixed with 250 g water at 90oC. 8 A 100 g sample of a liquid with a specific heat of 2 Determine the final temperature of the mixture, J/g·oC absorbs 4000 J of heat energy. If the sample assuming everything is liquid water in the end. started at 30oC, what is the final temperature? No phase change took place. 6 How many joules are required to heat 50. g of o aluminum (c = 0.9 C) from 68oC to 78oC? 9 its own heat capacity (C) which is the amount of heat it must absorb to change its temperature by one degree. It is defined as C = q/ T. When 1.50 g of methane burns in a bomb calorimeter (C = 11.3 kJ/oC) the temperature raises by 7.3oC. What is the heat of combustion, in kJ/g, for CH4 based on this? 7 A 30.0 g sample of a liquid at 280. K is mixed with 50.0 g of the same liquid at 330. K. Calculate the final temperature of the mixture, assuming no heat is lost to the surroundings. 10 The heat capacity of a bomb calorimeter was determined by burning 6.79 g of CH4 ( Hrxn = -802 kJ/mol CH4). The temperature increased by 10.8oC. What is the heat capacity of the bomb, in kJ/oC? 11 Match the following terms with the definitions below. endothermic system calorimetry exothermic heat positive energy negative Joule a. The capacity to do work ......................................................................................................... _____________ b. Energy transferred due to temperature differences .............................................................. _____________ c. The experimental measurement of heat flow ........................................................................ _____________ d. The part of the universe under study, which exchanges energy with the surroundings ....... _____________ e. When a system absorbs heat, the sign is _______________ and the process is................... _____________ f. When a system releases heat, the sign is _______________ and the process is ................. _____________ g. The SI unit of heat (equal to 1 N m) is named after a famous physicist and brewer, James . _____________ 5 67oC 6 450 J 7 311 K 8 50oC 9 -55 kJ/g 10 31.5 kJ/oC 11 see textbook 6.2 video AP chem. Thermochemistry, Solids & Liquids Name ____________________ (lesson 2 AIM: How do we measure heats of reaction?) Write the complete thermochemical equation for the combustion of methane: 12 What amount of heat is released when 5.8 g of methane burns? ( Hcomb for CH4 = -890 kJ/molrxn) H for a reaction is the same whether it occurs in one step or several. 14 H2 + ½ O2 H2O N2O5 + H2O 2 HNO3 ½ N2 + (3/2) O2 + ½ H2 2 N2 + 5 O2 2 N2O5 HNO3 H = -285.8 kJ/molrxn H = -76.6 kJ/molrxn H = -174.1 kJ/molrxn H= 13 The thermite reaction (below) uses iron(III) oxide and aluminum to make molten iron. What mass of iron is produced if a thermite reaction releases 25 kJ of heat? 2 Al + Fe2O3 2 Fe + Al2O3 H = -850 kJ/molrxn The sign changes if the reaction is _________________. Multiply H by a factor if the ________________ change. 15 C + O2 CO2 H2 + ½ O2 H2O C6H12O6 + 6O2 6 CO2 + 6H2O 6 C + 6 H2 + 3 O2 C6H12O6 H = -393.51 kJ/molrxn H = -285.83 kJ/molrxn H = -2803.02 kJ/molrxn Germain H= Hess HEATS OF FORMATION show the enthalpy change for the formation of 1 mole of a compound from elements. C(s) + 2 H2(g) CH4(g) = ________ H2(g) + ½ O2(g) H2O(g) = ________ = np (products) - nr (reactants) C(s) + O2(g) CO2(g) = ________ Use heats of formation to calculate the heat of reaction for each reaction below: 16 CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) 17 liquid methanol methane gas + oxygen gas BOND ENERGIES 18 Draw structures for CH4 + 2 O2 CO2 + 2 H2O & calculate the H: H C N O S F Cl Br I C=C C=N C=O C=S H 436 C 414 347 612 615 715 477 Single bond enthalpy (kJ/mol) N O S F 389 464 339 565 293 351 259 485 159 222 -272 138 -184 226 285 153 Multiple bond enthalpy (kJ/mol) N=N 418 N=O 607 O=O 498 S=O 498 Cl 431 331 201 205 255 255 243 Br 368 276 243 201 213 255 218 193 I 297 218 -201 -277 209 180 151 820 890 1075 941 AP chem. Thermochemistry, Solids & Liquids PROBLEM SET 6.2 19 H for: 2 B + 3 H2 B2H6 given: 24 2 B + 3/2 O2 B2O3 H = 1273 kJ H2 + O2 B2H6 + 3 O2 B2O3 + 3 H2O(g) H = 2035 kJ H2 + ½ O2 H2O(l) H = 286 kJ H2O(l) H2O(g) H = +44 kJ Name ____________________ H for the reaction: H2O2 Bond energy (kJ/mol) H H 432 H O 459 O O 207 O=O 494 25 (a) At STP, how many L H2(g) will release 550 kJ in: H2(g) + ½ O2(g) H2O(g) H = -286 kJ/molrxn? 20 Use heats of formation values (below) along with the reaction below to calculate of C6H6: 2 C6H6 + 15 O2 12 CO2 + 6 H2O(l) H = -6534 kJ (b) At STP, how many L CH4(g) will release 550 kJ in: CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g), H = -891 kJ/molrxn? Use values (below) to find Hrxn for each reaction: 21 4 NH3(g) + 7 O2(g) 4 NO2(g) + 6 H2O(l) 26 Given the thermochemical equations below: 2 C8H18(l) + 25 O2(g) 16 CO2(g) + 18 H2O(l) H = -10900 kJ H2(g) + ½ O2(g) H2O(g) = -286 kJ 22 2 Al(s) + Fe2O3(s) 23 2 CH3OH(l) + 3 O2(g) Al2O3(s) + 2 Fe(s) How many L of gaseous H2 (at 1.0 atm and 25oC) are required to furnish the same amount of energy as 80. L of liquid gasoline, C8H18 (d = 0.74 g/mL)? 2 CO2(g) + 4 H2O(l) 19 +36 kJ 20 45 kJ/mol 21 -1396 kJ 22 -850. kJ 23 -1454 kJ 24 -199 kJ 25 (a) 43 L H2 (b) 14 L CH4 26 240,000 L H2(g) 6.3 AP chem. Thermochemistry, Solids & Liquids Name ____________________ (lesson 3 AIM: What causes substances do have different forces of attraction?) 27 DEMO: Consider the following substances. Which has the highest melting point? Which has the lowest? aluminum metal glycerol glass graphite wax olive oil salt TYPES OF SOLIDS network covalent solids metallic solids ionic solids INTERMOLECULAR FORCES (only for molecular compounds!) 1. Dispersion forces 2. Dipole forces molecular solids 3. H-bonding 28 For each structure below, state its most significant intermolecular force (IMF): H H H O H b.p. = 100oC H C C O H H H b.p. = 78oC H H H H H C C O C C H H H H H b.p. = 35oC 29 Draw a structure for each and state the significant IMFs. Pick two and explain the osbserved boiling points. b.p. = -33oC 77oC -100oC 61oC 167oC -79oC -64oC 65oC NH3 CCl4 BF3 CHCl3 PCl5 CO2 SF6 CH3OH video AP chem. Thermochemistry, Solids & Liquids Name ____________________ PROBLEM SET 6.3 30 Identify the most important types of intermolecular / interparticle forces present in each of the following: HCl HF CaCl2 CH4 CO NaNO3 31 TYPES OF SOLIDS Use the following terms to complete the paragraph below: electrons ionic network metallic amorphous molecular crystalline Ice, iodine, and salicylic acid are all covalent solids, and can thus be classified as __________ solids. NaCl, KNO3, and CuSO4, on the other hand, are __________ solids, and as such have ionic forces of attraction which are much stronger than any of the intermolecular forces. Copper, iron and tungsten are all examples of _______ solids, and can conduct heat and electricity due to their mobile ____________. Quartz, diamond and graphite are common examples of ____________ solids, and have a continuum of covalent bonds. Solids such as glass and wax with highly disordered structures are called _______________ solids, as opposed to solids such as diamond, salt and ice, which have highly ordered structures, and are classified as _____________ solids. 32 ALLOYS & SEMICONDUCTORS interstitial quartz SiO2 Use the following terms to complete the paragraph below: graphite semiconductor glass substitution diamond Alloys in which atoms are replaced by a different atom of similar size (such as in brass, sterling silver, & pewter) are known as ___________________ alloys. Alloys in which additional atoms occupy spaces between atoms (such as in steel) are known as ____________________ alloys. The hardest known natural substance is ________________. This differs greatly from the more common allotrope of carbon, ________________, which, unlike diamond, can conduct due to its overlapping pi electrons. Silicon-based solids are primarily composed of silica, which is _____. The crystalline form of silica is _________, and the amorphous form of silica is ______. Many silicon compounds are manufactured to contain sites in which a silicon atom is replaced with an atom having more or less valence electrons (such as an arsenic atom). This creates a ______________________, which has an intermediate conductivity, in between that of a pure metal conductor, or a perfect insulator. 33 ALLOYS homogeneous mixtures that behave like metals Steel is an alloy that exhibits both interstitial and substitutional properties. It consists of iron with a small amount of carbon and chromium to make the steel less likely to rust. Chromium atoms react with oxygen in the air to form a nonreactive layer of chromium oxide on the surface, preventing further oxidation of underlying iron. Which of the following diagrams best shows a particle-level view of a surface section and an interior section of the alloy? A B C D (a) (b) (c) Fun fact: Samurai swords consist of different types of steel (iron and carbon). The core is made of lowcarbon steel, and the outer layer is made of high-carbon steel. The carbon gives it much more strength than the iron would have by itself. 6.4 AP chem. Thermochemistry, Solids & Liquids Name ____________________ (lesson 4 AIM: How do forces of attraction affect the physical properties of substances?) STRONG FORCES OF ATTRACTION?... high mp, high bp, low Pvap, high surf ten! PHASE DIAGRAMS Triple point: Critical point: VAPOR PRESSURE Pvap temp CALCULATING CHANGES IN BOILING POINT / VAPOR PRESSURE 34 At what temperature, in oC, would water boil at when the pressure is 23.8 torr, Hv = 43.9 kJ/mol for water? video AP chem. PROBLEM SET 6.4 Thermochemistry, Solids & Liquids 35 In each pair, which substance is expected to have the higher boiling point? Name ____________________ 37 Draw a structural formula for each of the following and determine which should have the highest boiling point. Briefly justify your reasoning. a. HF or HCl CH3CH2OH b. HCl or LiCl CH3OCH3 c. C5H12 or C6H14 CH3CH2CH3 d. Br2 or F2 36 Answer the following. Briefly justify your reasoning. a. Which has the higher boiling point: HBr or Kr b. Which has the higher freezing point: NaCl or HF c. Which has the lower vapor pressure at 25oC: Br2 or I2 d. Which has the lower boiling point: CH3CH3 or CH3CH2CH3 e. Which has the higher boiling point: HCl or HBr f. Which has the lower vapor pressure at 25oC: CH3CH2CH3 or CH3COCH3 38 LIQUIDS Use the following terms to complete the paragraph: viscosity adhesive surface convex cohesive capillary temperature _____________________, which typically decreases with an increase in ___________________. The rising of a attraction between molecules and a container, also known as __________________ forces. These differ from the attractive forces which exist between similar molecules, known as _______________________ forces. A column of mercury in a glass tube, for example, gives a ___________ meniscus because its cohesive forces for itself are stronger than its adhesive forces for the glass. 39 PRACTICE AP QUESTION White gold is a common substitutional alloy of gold (atomic radius = 135 pm) and palladium (atomic radius = 140 pm). Imagine a ring made of white gold that is 75 mole % gold and 25 mole % palladium. Draw a particle-level diagram of the alloy consisting of 12 atoms total. Use empty circles for gold and shaded circles for palladium. 40 PRACTICE AP QUESTION Consider nonane and 2,3,4-trifluoropentane, shown below, with their respective boiling points. H H H H H H H H H H F F F H H C C C C C C C C C H H C C C C C H H H H H H H H H H H H H H H 151oC b.p. = (a) What type of intermolecular forces are present in each compound? b.p. = 89oC (b) Explain the difference in boiling points. 6.5 AP chem. 1. 2. 3. 4. 6. 9. 10. From the table below, determine the enthalpy change for the reaction: 2 H2(g) + O2(g) 2 H2O(g) Bond energy (kJ/mol) H H 436 O=O 499 H O 464 a. 0 kJ b. 485 kJ c. -485 kJ d. 463 kJ 11. e. 443 kJ Name ____________________ If 10 g of a liquid at 300 K is heated to 350 K by adding 6 kcal, what is the specific heat of the liquid? a. 6 cal/goC b. 12 cal/goC c. 60 cal/goC d. 600 cal/goC e. 120 cal/goC Given that the reaction below has a change in enthalpy of -889.1 kJ, calculate the heat of formation of one mole of CH 4. CH4 + 2 O2 CO2 + 2 H2O Hof of H2O = -285.8 kJ/mol Hof of CO2 = -393.3 kJ/mol a. -210.0 kJ/mol b. -107.5 kJ/mol c. -76.0 kJ/mol d. +76.0 kJ/mol e. +210.0 kJ/mol When 58 g H2O (c = 4.18 J/g°C) is heated from 275 K to 365 K, a. absorbs 21,820 J b. absorbs 377 J c. releases 5220 J d. absorbs 242 J e. releases 90 J Given the equation: C2H4 + 3 O2 2 CO2 + 2 H2O(g), H = -1323 kJ, what is the value of H if liquid water is made instead of water vapor? (Note: H2O(g) H2O(l), Hv = -44 kJ) a. -1235 kJ b. -1279 kJ 12. A student uses a coffee-cup calorimeter in an experiment. The cup is c. -1323 kJ weighed, filled halfway with warm water then weighed again. The d. -1367 kJ temperature of the water was measured, and some ice cubes from a e. -1411 kJ 0oC ice bath were added to the cup. The mixture was gently stirred as the ice melted, and the lowest temperature reached by the water was The enthalpy of formation for potassium chloride is given by: recorded. The cup and its contents were weighed again. The purpose of a. K(g) + ½Cl2(g) KCl(g) weighing the cup and its contents again at the end of the experiment b. K+(g) + Cl-(g) KCl(s) c. d. e. 5. Thermochemistry, Solids & Liquids From the given data, determine the heat of formation of one mole of carbon dioxide in kJ/mol: 2 C + O2 2 CO H = -220 kJ 2 CO + O2 2 CO2 H = -560 kJ a. (-220) + (-560) b. ½[(-220) + (-560)] c. (-220) (-560) d. ½[(-220) (-560)] e. - (-220) + (-560) 2K(s) + Cl2(g) K(s) + ½Cl2(g) K+(g) + Cl-(g) 2KCl(s) KCl(s) KCl(g) For which of these processes is H expected to be negative? i. temperature increases when CaCl2 dissolves in water ii. steam condenses to liquid water iii. water freezes iv. dry ice sublimes a. iv only b. i, ii and iii c. i only d. ii and iii only e. i and ii only Which has a value of Hof 0? a. F2(g) b. Br2(g) c. I2(s) d. C(s, graphite) e. N2(g) 7. Which equation shows the formation of CH3OH(l) from its elements? a. CH3OH(l) + (3/2) O2(g) CO2(g) + 2 H2O(l) b. CH3OH(l) + (3/2) O2(g) CO2(g) + 2 H2O(g) c. 2 CH3OH(l) + 3 O2(g) 2 CO2(g) + 4 H2O(l) d. CH3OH(l) C(s) + 2 H2O(l) e. C(s) + 2 H2(g) + ½ O2(g) CH3OH(l) 8. 16 g H2O freezes at 273 K. What amount of heat is involved? ( Hfus = 6.02 kJ/mol for H2O) a. -16 J b. -4368 J c. -18,258 J d. -350 J e. -5351 J a. b. c. d. e. mass of ice added mass of the thermometer mass of water that evaporated mass of water that was cooled true mass of the calorimeter cup 13. Gaseous H2 and F2 combine in the reaction below to form HF with an enthalpy change of -540 kJ. What is the value of the heat of formation of one mole of HF(g)? H2(g) + F2(g) 2 HF(g) a. -1080 kJ/mol b. -540 kJ/mol c. -270 kJ/mol d. +270 kJ/mol e. +540 kJ/mol 14. Based on the information below, what is H for the reaction: CH4(g) + 2O2(g) CO2(g) + 2H2O(l) H = ? C(s) + 2H2(g) CH4(g) H = x C(s) + O2(g) CO2(g) H = y H2(g) + ½O2(g) H2O(l) H = z a. x+y+z b. x + y z c. z + y 2x d. 2z + y x e. 2z + y 2x 15. The densities of certain compounds are greater as liquids than as solids (e.g. H2O). This means that increasing the pressure will result in a. a solid becoming a liquid. b. a liquid becoming a solid. c. lowering of the critical temperature. d. elevation of the freezing temperature. e. lowering of the triple point. AP chem. Thermochemistry, Solids & Liquids 16. The liquefied hydrogen halides (HX) have the normal boiling points given below. The relatively high boiling point of HF can be explained: HF = +19 oC HCl = -85 oC HBr = -67 oC HI = -35 oC a. HF gas is more ideal b. HF is the strongest acid c. HF molecules have a smaller dipole moment d. HF is much less soluble in water e. HF molecules tend to form hydrogen bonds Name ____________________ 22. The boiling points of He, Ne, Ar, Kr, and Xe increase in that order. What accounts for this? a. The London (dispersion) forces increase b. The hydrogen bonding increases c. The dipole-dipole forces increase d. The chemical reactivity increases e. The number of neighbors increases 23. The graph here shows the temperature of a pure substance as it is heated at a constant rate. The substance is boiling point at 17. The normal boiling point of the substance in the phase diagram here is 24. Which likely has the highest melting point? a. S8 b. I2 c. SiO2 d. SO2 e. C6H6 a. b. c. d. e. -15 oC -10 oC 140 oC Greater than 140 oC Not determinable from the graph 18. The critical temperature is the a. T at which Pvap of the liquid is equal to the external pressure b. T at which the Pvap of the liquid is equal to 760 mmHg c. T at which the solid, liquid, and vapor phases are all in equilibrium d. T at which liquid and vapor phases are in equilibrium at 1 atm e. Lowest T above which a substance cannot be liquefied at any applied pressure 25. In methane, the forces between neighboring molecules are a. ionic bonds b. covalent bonds c. hydrogen bonds d. ion-dipole forces e. London (dispersion) forces 26. Two closed containers of acetone are below, each with same volume of acetone at the same temperature. The vapor pressure of the acetone is a. b. c. d. e. higher in 1 because the surface area of the liquid is greater higher in 1 because the volume of the vapor is greater lower in 1 because the level of the liquid is lower the same in both because the volume of the liquid is the same the same in both because the temperature is the same 19. Which of the following is true at the triple point of a pure substance? a. The Pvap of the solid phase equals the Pvap of the liquid phase. b. The T is always 0.01 K lower than the normal melting point. c. The liquid and gas phases of the substance always have the same density and are therefore indistinguishable. 27. Which explains why the boiling point of CCl4 is higher than that of CF4? d. The solid phase always melts if P increases at constant T. a. The C Cl bonds in CCl4 are less polar than the C F bonds in CF4 e. The liquid phase always vaporizes if P increases at constant T. b. The C Cl bonds in CCl4 are weaker than the C F bonds in CF4 c. The mass of the CCl4 molecule is greater than that of the CF4 20. Which of the following actions would be likely to change the boiling molecule point of sample of a pure liquid in an open container? d. The electrons in CCl4 are more polarizable than in CF4 i. Placing it in a smaller container e. The bonds in CCl4 are covalent, whereas ionic in CF4 ii. Increasing the number of moles of the liquid in the container iii. Moving the container and liquid to a higher altitude 28. Which exhibits H-bonding? a. i only a. CH2F2 b. ii only b. N2H4 c. iii only c. CH3OCH3 d. ii and iii only d. C2H4 e. ii, ii, and iii e. CH 2 2 21. The phase diagram for a pure substance is shown below. Which point 29. on the diagram corresponds to the equilibrium between the solid and a. liquid phases at the normal melting point? b. c. d. e. Solid silver melts Solid potassium chloride melts Solid carbon (graphite) sublimes Solid iodine sublimes Glucose dissolves in water 30. Which shows a correct vapor pressure trend? a. CH4 < C2H5OH < C2H5OC2H5 < Ne b. Ne < CH4 < C2H5OC2H5 < C2H5OH c. C2H5OH < C2H5OC2H 5 < CH4 < Ne d. C2H5OC2H5 < C2H5OH < CH4 < Ne e. C2H5OC2H5 < C2H5OH < Ne < CH4 ANSWERS 1. B 2. C 3. E 4. D 5. B 6. B 7. E 8. E 9. B 10. C 11. A 12. A 13. C 14. D 15. A 16. E 17. C 18. E 19. A 20. C 21. C 22. A 23. D 24. C 25. E 26. E 27. D 28. B 29. C 30. C AP Chem Thermochemistry Practice Test 06 Name ____________________ FREE RESPONSE – Answer the following in the space provide below. You must show work for full credit. The boiling points, dipole moments, and polarizabilities of three hydrogen halides are given in the table below. A) (5 points) Based on the data in the table, what type of intermolecular force among the molecules HCl, HBr, and HI is able to account for the trend in boiling points? Justify your answer. B) (5 points) Based on the data in the table, a student predicts that the boiling point of HF should be 174 K. The observed boiling point of HF is 293 K. Explain the failure of the student’s prediction in terms of the types and strengths of the intermolecular forces that exist among HF molecules. C) (5 points) A representation of five molecules of HBr in the liquid state is shown in Box 1 below. In Box 2, draw a representation of the same five molecules of HBr after complete vaporization has occurred. MULTIPLE CHOICE (5 points each) – Bubble the letter of the best answer to each question. 1. Which of the following arranges the molecules N2, O2, and 3. Which liquid possesses dipole-dipole intermolecular forces? F2 in order of their bond enthalpies (aka bond energies), a. F2(l) from least to greatest (aka weakest to strongest)? b. CH4(l) a. F2 < O2 < N2 c. CF4(l) b. O2 < N2 < F2 d. CH2F2(l) c. N2 < O2 < F2 d. N2 < F2 < O2 4. Which process is exothermic? a. condensation 2. Based on the information in the table below, which liquid, b. melting CS2(l) or CCl4(l), has the higher equilibrium vapor pressure c. sublimation at 25oC, and why? d. vaporization molar mass (g/mol) boiling point (oC) 5. A sample of a solid binary compound at room temperature CS2(l) 76 46.5 does not conduct electricity but becomes conductive CCl4(l) 154 76.7 when dissolved in water. Which type of interactions are a. CS2(l); it has stronger London dispersion forces likely found in this substance? b. CS2(l); it has weaker London dispersion forces a. Ionic bonds c. CCl4(l); it has stronger London dispersion forces b. Metallic bonds d. CCl4(l); it has weaker London dispersion forces c. Covalent bonds d. Hydrogen bonds AP Chem 6. 7. 8. 9. Thermochemistry At room temperature I2(s) is a molecular solid. Which of the following provides a characteristic of I2(s) with a correct explanation? a. It has a high melting point because it has weak intermolecular forces. b. It is hard because it forms a three-dimensional covalent network. c. It is not a good conductor of electricity because its valence electrons are localized in bonding and nonbonding pairs. d. It is very soluble in water because its molecules are polar. Name ____________________ 11. Use the thermodynamic information to find the mising ∆H: ½ N2(g) + ½ O2(g) → NO(g) ∆H = +90. kJ/molrxn ½ N2(g) + O2(g) → NO2(g) ∆H = +35 kJ/molrxn 2 NO2(g) → N2O4(g) ∆H = -60 kJ/molrxn 2 NO(g) + O2(g) → N2O4(g) ∆H = ? kJ/molrxn a. -170. kJ b. -115 kJ c. +115 kJ d. +170. kJ 12. Determine the enthalpy change when 5.00 g of Fe2O3(s) (MM = 159.70 g/mol) reacts with excess Al(s) in the reaction: Fe2O3(s) + 2 Al(s) → Al2O3(s) + 2 Fe(l) 5.00 A student mixes a 10.0 mL sample of 1.0 M NaOH(aq) with a. -825.2 4 kJ Enthalpy of formation, ∆Hof 159.70 a 10.0 mL sample of 1.0 M HCl(aq) in a polyester container. 5.00 o b. -837.8 kJ Fe2O3(s) -825.5 kJ/mol The temperature of the solutions before mixing was 20.0 C. 159.70 2 x 5.00 -1675.7 kJ/mol If the final temperature of the mixture is 26.0oC, what is c. -837.8 kJ Al2 O3(s) 159.70 the experimental value of ∆Hrxn? (Assume the solution Fe(l) +12.4 kJ/mol 159.70 d. -825.2 4 kJ mixture has a specific heat of 4.2 J/(g•K) and a density of 5.00 1.0 g/mL.) a. -50. kJ/molrxn 13. The critical temperature of water is the… b. -25 kJ/molrxn a. temperatrue at which solid, liquid, and gaseous water 4 c. -5.0 x 10 kJ/molrxn coexist. d. -5.0 x 102 kJ/molrxn b. tempearture at which water vapor condenses. c. maximum temperature at which liquid water can When water is added to a mixture of Na2O2(s) and S(s), a exist. redox reaction occurs according to: d. minimum temperature at which water vapor can exist. 2 Na2O2 + S + 2 H2O → 4 NaOH + SO2 ∆H298 = -610 kJ/molrxn 14. Use bond energies to calculate ∆H for the reaction: Two trials are run, using excess water. In the first trial, 7.8 H2 + O2 → H2 O2 Bond energy (kJ/mol) g of Na2O2 (78 g/mol) is mixed with 3.2 g S (32 g/mol). In a. -521 kJ H—H 432 the second trial, 7.8 g Na2O2 is mixed with 6.4 g S. The b. -486 kJ H—O 459 Na2O2 and S react as completely as possible. Both trials c. -199 kJ O—O 207 yield the same amount of SO2. Which of the following d. +199 kJ O=O 494 identifies the limiting reactant and the heat released, q, for the two trials at 298 K? 15. Under certain conditions CO2 melts rather than sublimes. a. Limiting reactant = S q = 30. kJ To which transition in the phase diagram does this change b. Limiting reactant = S q = 61 kJ correspond? c. Limiting reactant = Na2O2 q = 30. kJ a. A → B d. Limiting reactant = Na2O2 q = 61 kJ b. A → C c. B → C Which reaction correctly depicts the enthalpy change of d. C → B formation of sodium carbonate? 3 16. Which liquid has the highest vapor pressure at 25oC? a. 2 Na(s) + C(s) + O2(g) → Na2CO3(s) 2 a. Butane, C4H10 b. Glycerol, b. Na2O(s) + CO2(g) → Na2CO3(s) H H H H C3H5(OH)3 c. 2 Na+(aq) + CO32-(aq) → Na2CO3(s) d. H C C C C H H H H H 2 Na+(aq) + 2 OH-(aq) + CO2(aq) → Na2CO3(s) + H2O(l) 10. The reaction below takes place in a rigid, insulated vessel that is initially at 600 K: CH3OH(g) → CO(g) + 2 H2(g); ∆H = +91 kJ/molrxn Which of the following statements about the bonds in the reactants and products is most accurate? a. The sum of the bond enthalpies of the bonds in the reactant is greater than the sum of the bond enthalpies of the bonds in the products. b. The sum of the bond enthalpies of the bonds in the reactant is less than the sum of the bond enthalpies of the bonds in the products. c. The length of the bond between carbon and oxygen in CH3OH is shorter than the length of the bond between carbon and oxygen in CO. d. All the bonds in the reactant and products are polar. c. Octane, C8H18 H H H H H H H H H C C C C C C C C H H H H H H H H H H H H H C C C H OH OH OH d. Propanol, C3H7OH H H H H C C C OH H H H 17. Of the species below, the lowest melting points overall occur for members of which class of solids? a. Ionic c. Metallic b. Molecular d. Network covalent 18. (EXTRA CREDIT) An example of an interstitial alloy is shown here. Compared with the pure metal, how would you expect the properties of the alloy to vary? a. The alloy has higher malleability and lower density. b. The alloy has lower malleability and higher density. c. The alloy has higher malleability and higher density. d. The alloy has lower o’malleability and lower density. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. A B D A A C A C A A A A C C A A B B






