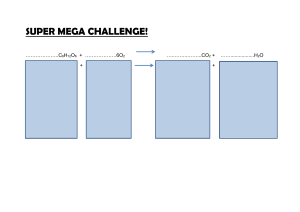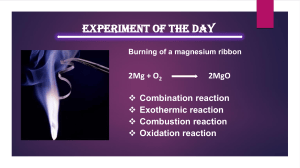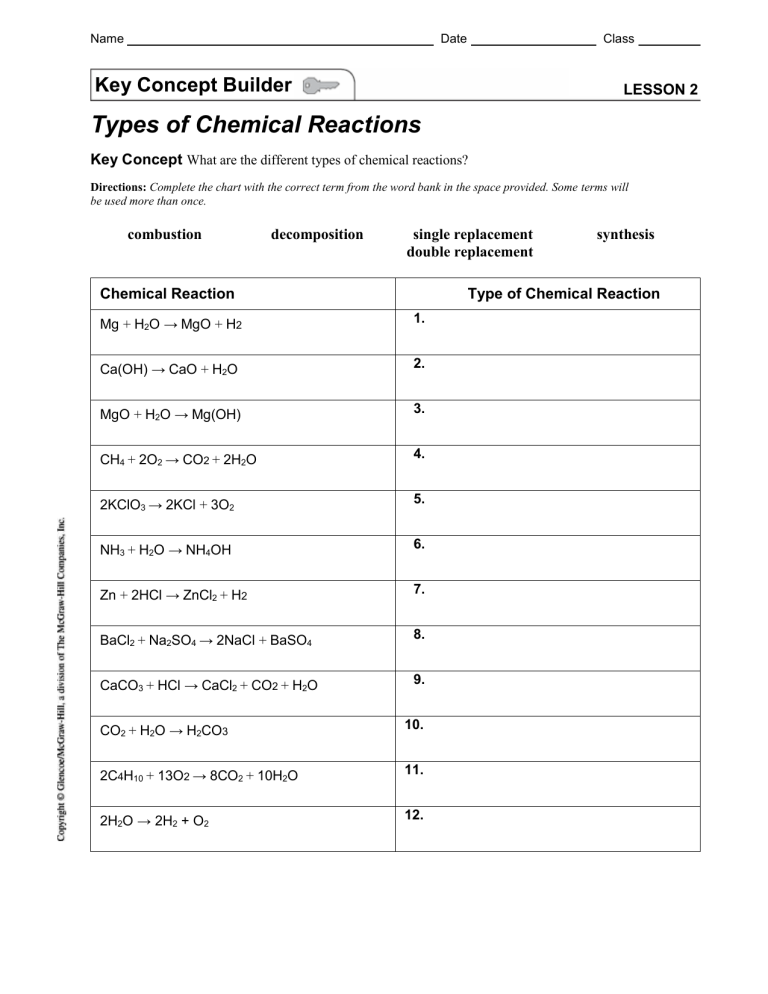
Name Date Class Key Concept Builder LESSON 2 Types of Chemical Reactions Key Concept What are the different types of chemical reactions? Directions: Complete the chart with the correct term from the word bank in the space provided. Some terms will be used more than once. combustion decomposition single replacement double replacement Chemical Reaction synthesis Type of Chemical Reaction Mg + H2O → MgO + H2 1. Ca(OH) → CaO + H2O 2. MgO + H2O → Mg(OH) 3. CH4 + 2O2 → CO2 + 2H2O 4. 2KClO3 → 2KCl + 3O2 5. NH3 + H2O → NH4OH 6. Zn + 2HCl → ZnCl2 + H2 7. BaCl2 + Na2SO4 → 2NaCl + BaSO4 8. CaCO3 + HCl → CaCl2 + CO2 + H2O 9. CO2 + H2O → H2CO3 10. 2C4H10 + 13O2 → 8CO2 + 10H2O 11. 2H2O → 2H2 + O2 12. Chemical Reactions and Equations 39