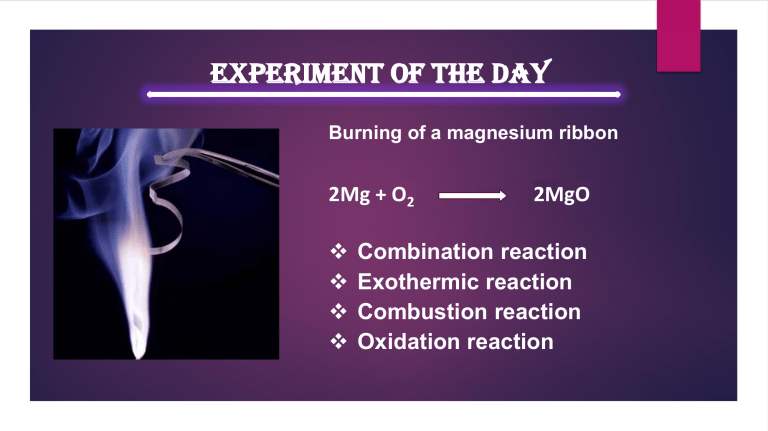
Experiment of the Day Burning of a magnesium ribbon 2Mg + O2 ❖ ❖ ❖ ❖ 2MgO Combination reaction Exothermic reaction Combustion reaction Oxidation reaction Experiment of the Day Decomposition of Ferrous sulfate 2FeSO4(s) Heat Fe2O3(s) + SO2(g) + SO3(g) Experiment of the Day Reaction of Zinc with Hydrocloric acid Zn (s) +2HCl (aq) ZnCl2 (aq) + H2 (g) Zn (s) + H2SO4 (aq) ZnSO4 (aq) + H2 (g) ❖ Displacement reaction ❖ Exothermic reaction Set up of experiment Experiment of the Day Reaction of lead (II) nitrate with potassium iodide ❖ Double displacement reaction ❖ Precipitation reaction Pb(NO3)2 (aq)_+ 2 KI (aq) PbI2 (s) + 2 KNO3(aq) Experiment of the Day Reaction of sodium metal with water Sodium + Water 2Na + H2O Na2O + H2O 2Na(s) +2 H2O(l) Sodium hydroxide + Hydrogen Na2O + H2 2NaOH 2NaOH(aq) + H2 (g) Experiment of the Day ❖ ಪ್ರಬಲ ಆಮ್ಲ ಮ್ತ್ತು ಪ್ರತ್ಯಾಮ್ಲಗಳು ಹೆಚ್ಚಿನ ವಿದ್ತಾತ್ುನತು ಹರಿಸತತ್ುವೆ. ❖ ದ್ತಬಬಲ ಆಮ್ಲ ಮ್ತ್ತು ಪ್ರತ್ಯಾಮ್ಲಗಳು ಕಡಿಮೆ ವಿದ್ತಾತ್ುನತು ಹರಿಸತತ್ುವೆ. Electric Conductivity Of Acid Experiment of the Day Reaction of magnessium metal with water magnessium + Water Mg + H2O MgO + H2O Mg(s) +2 H2O(l) magnessium hydroxide + Hydrogen MgO + H2 Mg(OH)2 Mg(OH)2(aq) + H2 (g) Experiment of the Day Electric decomposition of water 2H2O (aq) current 2H2 (g)+ O2 (g) Experiment of the Day Testing carbon dioxide gas Ca(OH)2(aq) + CO2 (g) CaCO3 (s) + H2O (l) + CO2 (g) CaCO3(s) + H2O(l) Ca(HCO3) 2 (aq) Experiment of the Day ❖ ಲವಣಗಳು ನೀರಿನಲ್ಲಲ ವಿಲ್ಲನವಯಗತತ್ುವೆ. ❖ ಲವಣಗಳು ದ್ರವಿಸಿದ್ ಸಿಿತಿ ಅಥವಯ ದ್ಯರವಣ ಸಿಿತಿಯಲ್ಲಲ ವಿದ್ತಾತ್ನತು ಹರಿಸತತ್ುವೆ. Experiment of the Day Reaction of sodium sulfate with barium chloride ❖ Double displacement reaction ❖ Precipitation reaction Na2 SO4 (aq)_+ BaCl2 (aq) BaSO4 (s) + 2NaCl (aq) Experiment of the Day Decomposition of lead (II) nitrate 2Pb(NO3)2(s) Heat 2PbO(s) +4NO2(g) + O2(g) Experiment of the Day Crystalisation and decrystalisation of copper sulfate CuSO4 5H2O