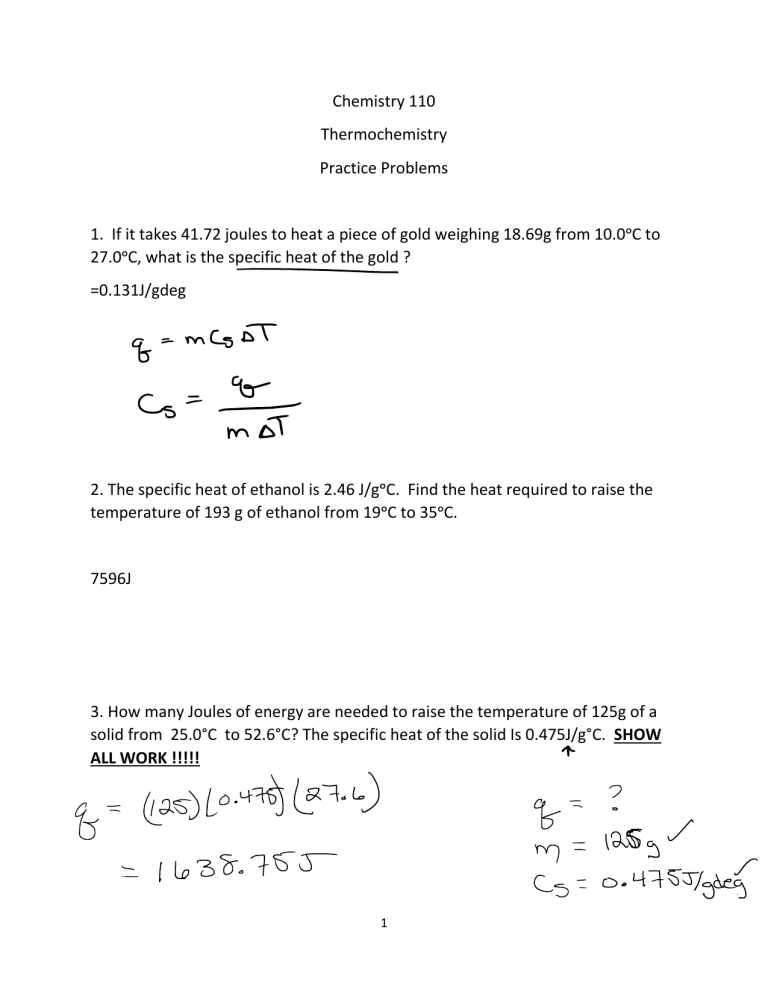
Chemistry 110 Thermochemistry Practice Problems 1. If it takes 41.72 joules to heat a piece of gold weighing 18.69g from 10.0ᵒC to 27.0ᵒC, what is the specific heat of the gold ? =0.131J/gdeg 2. The specific heat of ethanol is 2.46 J/gᵒC. Find the heat required to raise the temperature of 193 g of ethanol from 19ᵒC to 35ᵒC. 7596J 3. How many Joules of energy are needed to raise the temperature of 125g of a solid from 25.0°C to 52.6°C? The specific heat of the solid Is 0.475J/g°C. SHOW ALL WORK !!!!! 1 4. a. Calculate the ∆HRXN for the following balanced equation. 2 H2S (g) + 3 O2(g) → Chemical 2 H2O (l) + 2 SO2(g) ∆H H2S(g) -20.1 kJ/mole H2O(l) -285.8 kJ/mole SO2(g) -296.1 kJ/mole O2 (g) 0 kj/mol b. Is the above reaction endothermic or exothermic ? _____Exo___________ -1123.6 kJ 5. a. Calculate the ΔHᵒRXN for the following balanced equation. C2H5OH (l) + 3 O2(g) → Chemical 2 CO2 (g) + 3 H2O (l) ΔHᵒf C2H5OH (l) -228 kJ/mol CO2(g) -394 kJ/mol H2O (l) -286 kJ/mol O2 (g) 0 kj/mol b. Is the above reaction endothermic or exothermic ? ___________________ 2 6. The combustion of methane gas is represented by the reaction: CH4 (g) + 2 O2 → Calculate the ∆ H CO2(g) + 2 H2O (g) ° given the following information. rx Substance ∆Hf° CH4 - 74.8 kJ/mole O2 0 kJ/mole CO2 -393.5 kJ/mole H2O -241.8 kJ/mole b. Is the above reaction endothermic or exothermic ? ____exo____________ -802 KJ 7. Given the following information, calculate the ∆H for the target reaction. SHOW ALL WORK !!!!! Target: NO2(g) + 7 ⁄2H2(g) ---> 2H2O(l) + NH3(g) 3 Given: 1.) 2NH3(g) ---> N2(g) + 3H2(g) ΔH° = +92 kJ 2.) 1 ⁄2N2(g) + 2H2O(l) ---> NO2(g) + 2H2(g) ΔH° = +170 kJ 8. Given the following information, calculate the ∆H for the target reaction. SHOW ALL WORK !!!!! Target: 2 CH4(g) + 3 O2(g) ---> 2 CO(g) + 4 H2O(l) Given: 1.) CH4(g) + 2 O2(g) ---> CO2(g) + 2 H2O(l) ∆H°= -890.0 kJ 2.) 2 CO(g) + O2(g) ---> 2 CO2(g) ∆H°= -566.0 kJ 4 9. Calculate the ∆H for the following reaction: SHOW ALL WORK!!!!!!!!!!!! Target: 2C2H4(g) + H2O(l) → C4H9OH(l) Given: 1. 2CO2(g) + 2H2O(l) → C2H4(g) + 3O2(g) ∆H = +1411.1 kJ 2. C4H9OH(l) + 6O2(g) → 4CO2(g) + 5H2O(l) ∆H = -1534.7 kJ 5