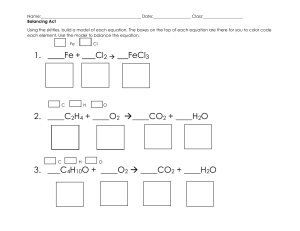
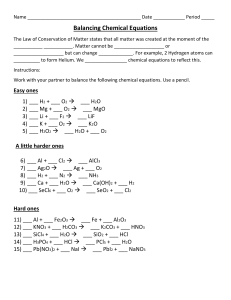
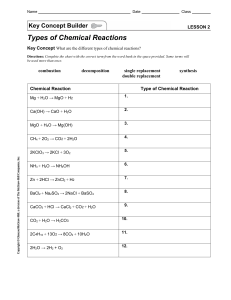
Balancing Redox Equations Using Oxidation Numbers Name& Date: Use the following Steps 1. Assign oxidation numbers to all atoms in the equation 2. Identify the atoms that change oxidation number with square brackets. 3. Balance the atoms that are undergoing ox/red with temporary coefficients 4. Determine the total change in oxidation state for the oxidizing and reducing agents. 5. Balance the two changes in oxidation state with an appropriate multiplication factor. 6. Balance the rest of the equation by inspection, but leave H and O until last. KCl + MnO2 + HNO3 + H2SO4 H3AsO3 → K2SO4 + → H3AsO4 + MnSO4 + NO + Cl2 + H2O H2 O Oxidation of isopropyl alcohol to acetone (draw organic structures above the formula) C3H8O + I2 + CrO3 + → HNO3 HBr + HBrO3 MnO4- + H+ + CrO2- + ClO- + → H2SO4 Cr2(SO4)3 + HIO3 + NO2 + → Br2 Cl- → Mn2+ OH- → CrO42- + + H2 O H2O +Cl2 + H2O Cl- + H2O C3H6O + H2O Senior Chem Name: Balancing Redox Reactions Using Half Reactions Note: A chemical equation is balanced only when both the mass and the charge are balanced. 1. The permanganate ion (MnO) reacts with the hydrogen sulphite ion (HSO) in different ways, depending on whether extra acid or base or nothing has been added to the reaction mixture. Balance the following equations, using the half reactions given. The spectator ions (e.g. Na+ and K+) have been left out to simplify things. a) Extra acid was added to the reaction mixture. Balance the following equation: MnO41- + HSO31- H+ Mn2+ + SO42- + H2O(l) Half reactions: MnO41- + 8H+ + 5e- . HSO31- + H2O(l) . Mn2+ + 4H2O(l) SO42- + 3H+ + 2e- b) Extra base was added to the reaction mixture. Balance the following equation: MnO + HSO + OH MnO + SO + H2O(l) Half reactions: MnO+ eHSO + 3OH MnO SO + 2H2O(l) + 2e- c) Nothing extra was added to the reaction mixture. Balance the following equation: MnO + HSO + OH MnO2(s) + SO + H2O(l) Half reactions: MnO HSO + 2H2O(l) + 3e+ 3OH SO MnO2(s) + 4OH + 2H2O(l) + 2e- 2. Write the reduction and oxidation half reactions for this process and balance the equation. Au3+ + IAu(s) + I2(s) 3. Use the half reactions given below to balance the following redox reaction: HNO2(aq) + Cr2O7+ H NO+ Cr+ H2O(l) Half reactions: NO+ 3H+ 2eHNO2(aq) + H2O(l) Cr2O7+ 14H+ 6e2Cr+ 7H2O(l) 4. Use the half reactions given below to balance each of the following redox reactions. This time the spectator ions are included in the given equation and will have to be balanced by inspection as a final step after you have added the half reactions together. a) K2Cr2O7(aq) + HCl(aq) KCl(aq) + CrCl3(aq) + Cl2(g) + H2O(l) Half reactions: Cr2O7+ 14H+ 6e2Cr+ 7H2O(l) Cl2(g) + 2e2Cl b) NaIO3(aq) + NaI(aq) + HCl(aq) Half reactions: IO+ 6H+ 5e2I NaCl(aq) + I2(s) + H2O(l) I2(s) + 3H2O(l) I2(s) + 2e- c) Cu(s) + HNO3(aq) Cu(NO3)2(aq) + NO(g) + H2O(l) Half reactions: Cu + 2eCu(s) NO + 4H+ 3eNO(g) + 2H2O(l) d) Cu(s) + HNO3(aq) Half reactions: Cu(NO3)2(aq) + NO2(g) + H2O(l) Cu+ 2e2NO+ 4H+ 2e- Cu(s) 2NO2(g) + 2H2O(l)