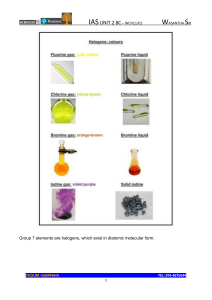
Group 7 – The Halogens Group 7: The halogens How does reactivity change as you descend Group 7? …………………………………………………………………………………………………………… ………………………………………………. Explain why. …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… What type of bonds do the halogens form? …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… Group 7 – The Halogens ● Displacement of halides with halogens What is the difference between a halogen and a halide? …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… A more reactive halogen can displace a less reactive halogen from a solution of one of its salts. Write a word and balanced symbol equation for the reaction of bromine with sodium iodide. …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… Write a word and balanced symbol equation for the reaction of chlorine with sodium bromide. …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… Write a word and balanced symbol equation for the reaction of iodine with sodium chloride. …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… Which of the following reactions will not occur as written? A. Cl2(aq) + 2NaBr(aq) → 2NaCl(aq) + Br2(aq) B. I2(aq) + 2KBr(aq) → 2KI(aq) + Br2(aq) C. Br2(aq) + 2LiI(aq) → 2LiBr(aq) + I2(aq) Group 7 – The Halogens ● Reaction of halogens with hydrogen Write a word and symbol equation for the reaction of hydrogen and chlorine? ……………………………………………………………………………………………………………………………………………………………. ……………………………………………………………………………………………………………………………………………………………. Write a word and symbol equation for the reaction of hydrogen and bromine? ……………………………………………………………………………………………………………………………………………………………. ……………………………………………………………………………………………………………………………………………………………. Summary questions 1. Fill in the blanks using words from the word bank Group 7 elements are also called the …………………… Fluorine, at the ……………………… of the group is the …………………. reactive, while iodine is much ………………………. reactive. They react with other non-metals to form compounds which have …………………….. bonds. With metals they react to form ………………………… compounds. Word bank: covalent, halogens, ionic, less, most, top. 2. Write a word and balanced symbol equation, including state symbols, for the reaction of: i) Potassium metal and iodine vapour …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… ii) Chlorine water and sodium iodide solution …………………………………………………………………………………………………………… ……………………………………………………………………………………………………………