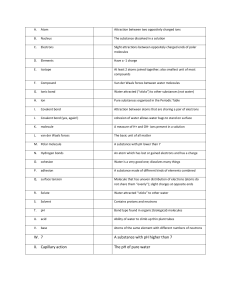
Two-dimensional representations: electron dot structures: pairs of electrons/single covalent bond represented by ·· e.g., H : H structural formulas: single covalent bond represented by – e.g., H – H Covalent molecules have single, double and triple bonds. Atoms can have a chainlike or ringlike structure, or branched chanins. Molecular formula may represent more than one compound because atoms may form more than one structure. Structural isomers: substances with same chemical formula but different structure (does not include rotations of bond electrons).