Презентация Microsoft PowerPoint (2)
advertisement

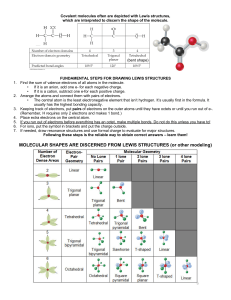
The theme of the lesson: Chemical bonds Learning objectives: • What is it? • How is it formed? • How to classify? When the electronegativities of two atoms are quite different from each other: One atom loses an electron (or electrons) The other atom gains an electron (or electrons) This results in an Ionic Bond. NaCl Crystal Lattice metal + nonmetal + 11 Na)2)8) 1 + 17 Cl)2)8) Electrostatic attraction 7 Covalent coupling is a connection that arises between atoms due to the formation of common electronic pairs By the degree of bias of common electronic pairs to one of the atoms bound by them, the covalent bond can be polar and nonpolar Covalent nonpolar connection (CNS) - form atoms of the same chemical element - non-metal (For example: H2, O2, O3). Covalent Polar Communication (KPS) - form atoms of different non-metals, differing in the values of electronegativity (For example: HCl, H2O). Metal bond It is a relationship in metals and alloys which are relatively free electrons between the metal ions in the metal crystal lattice Diagram metallic bond M° - nē ↔Mⁿ