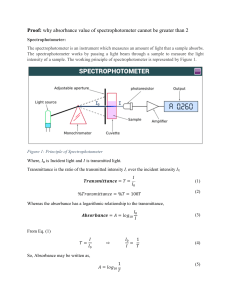
UNIVERSITY OF DAR ES SALAAM DEPARTMENT OF MOLECULAR BIOLOGY AND BIOTECHNOLOGY BN 240: PRACTICALS IN BIOCHEMISTRY PRACTICAL FIVE COLORIMETRIC DETERMINATION OF REDUCING SUGAR CONCENTRATION INTRODUCTION Carbohydrates are organic compounds that are either aldehydes or ketones and have many hydroxyl groups. Sugar can be detected in natural and processed foods and drinks by chemical and biochemical methods. Such detections can be useful for not just determining amount of sugar in food or drink damples, but also in determining validity of labels. Monosaccharides, the simplest carbohydrate units, are commonly known as 'simple sugars'. Two monosaccharides can form a glycosidic linkage by undergoing a condensation reaction, which yields a disaccharide. A disaccharide can be digested and broken down into its original monosaccharide units. In this experiment you will determine the amount of sugar or carbohydrate in a soft drink by spectrophotometric (colorimetric) method. The method is based upon the colour that forms when sugars reduce 3,5-denitrosalicylic acid (DNSA) to 3-amino-5-nitrosalicylic acid as shown in the equation. Hо H-C OH HỌC-H H-C он 0 OH OH ON + H-C-OH H-C-H OH OH OH O,N NH₂ + OH OH O HO OH OH OH 3,5-dinitrosalicylic Acid (Yellow) Glucose 3-amino-5-nitrosalicylic Acid (red-brown) Gluconic acid One problem with this method is that sucrose, or common table sugar, does not undergo this reaction with 3, 5-dinitrosalicylic acid. Therefore, the sucrose and complex carbohydrates must be broken down into simple sugars like glucose and this can either be biochemically using an enzyme invertase or chemically using an acid such as hydrochloric acid. In this experiment, boiling the sample with hydrochloric acid will be used do this. The pH is then adjusted to give a basic solution, under this condition simple sugars are good reducing agents. In addition, the sugars in soft drinks are at highly concentrated therefore dilutions must be done done before carrying out the analysis. These dilutions must be carried out quantitatively (in a precisely known manner) using volumetric flask. Some suggested dilutions include 1:100, 2:100, 3:100, 5:100 and 10:100, depending upon how much sugar is in your sample. One application of light absorption (spectroscopy) is to determine the concentration of a molecule that absorbs light. If a beam of monochromatic (single-wavelength) light of intensity lo enters a sample which absorbs some of the light, the beam of light emerging from the other side of the sample will have a reduced intensity I. The transmittance of the sample is then defined as The transmittance is often expressed as percent transmittance rather than a fraction. The transmittance can be measured using spectrophotometer by calculating the ratio of the two intensities. In this experiment, you will measure concentration of sugars using light absorbance measurements. Specifically you will test for the concentration of the coloured product formed by the reaction of glucose with 3,5-dinitrosalicylic acid. Since all of the glucose will be converted to the coloured product, the BeerLambert law can be used to determine sugar concentration. The amount of colour formed is timedependent. Therefore, once the 3,5-dinitrosalicylic acid is added to the test solution, a timer should be started and the absorbance measurements for all samples should be made at the same time after the addition. A time interval of 5 to 20 minutes will suffice. It is necessary to first prepare a calibration curve known as a Beer's Law Plot. To do this, a series of standards is prepared in which the concentrations of the absorbing species are precisely known. The absorbances of these standards are then measured in the spectrophotometer and a plot of absorbance versus concentration is prepared. This plot can then be used to relate the measured absorbance of any other sample containing the same absorbing species to the concentration of that species in the solution. According to Beer's law, the absorbance of a solution should be zero (100%T) if there is none of the absorbing species present. A blank solution containing all components of the solution except the sample is used to set the spectrophotometer to a reading of zero absorbance. Thus, if any other components of the mixture absorb light at the wavelength used, their effect will be cancelled out. Equipment and Reagents 6M HCI 2.5M NaOH 0.05 M 3,5-dinitrosalicylic acid 1000mg/L standard sucrose solution Soft drink to test (non-diet, not dark colored) Cuvettes, test tubes, test tube rack, test tube clamp, (2) 400 mL beaker, Mohr pipettes and bulb (5 and 10 mL), 25 mL volumetric pipette, (5) 100 mL volumetric flasks, Pasteur pipette and bulb, tissue paper. EXPERIMENTAL PROCEDURE A. Preparation of Sucrose Standard Solutions and Calibration Curve 1. Prepare the sucrose standards by suitable dilution of the stock solution. Concentrations in the range 100-1000 mg/L are suggested as a starting point. 2. Make a 2:10 dilution as follows: Pipette 2.0 mL of the stock solution into a clean 10 mL test tube, and add distilled water to calibration mark of 10 ml Cover the flask and shake well to mix. In a similar fashion, prepare 4:10, 6:10 and 8:10 dilutions 3. Prepare five standards (the original stock solution and the four dilutions) as follows: Pipette 2.00 mL of each sucrose standard solution (five standards), into test tubes. Add 2.00 mL of 6 M HCI into each test tube and place in a boiling water bath for 10 minutes. Remove the test tubes and carefully pipette 8.00 mL of 2.5M NaOH, 2.00 mL of 0.050 M 3,5dinitrosalicylic acid (DNSA) into each test tube. As soon as the DNSA is added, cover the tube and, shake to mix the solution thoroughly. Place the tube in a boiling water bath for 5 minutes. Begin timing when the tube is placed in the hot water; each tube must remain in the boiling water for the same amount of time. Remove each test tube from the boiling-water bath at the proper time and quickly place it in an icewater bath for 10 minutes. Pour some of the blank solution (distilled water) into a clean, dry cuvette (fill halfway), and place it in the spectrophotometer. Take measurement of the standard solution and record the absorbance in triplicate for each dilution. 4. Draw a rough graph of Absorbance versus Concentration of sucrose. If the data are not linear, repeat the exercise. It is important to heat each solution for the same amount of time when developing the colour You may wish to use the Excel spreadsheet to perform a linear least-squares regression on the data to determine the slope and intercept of the line. This is your Beer's Law Calibration Plot. B. Preparation of Unknown Sample Solutions Prepare dilutions of your beverage as follows: 0.1:100, 0.2:100, (0.4:100) 0.6:100 and 0.7:100 mL Treat 2.00 mL aliquots of the diluted samples in the same manner that you did for the standards. Carry out the measurement in triplicate for each dilution. Be sure to use the same time interval after adding the DNSA that you used for the standards. Record the absorbance for the diluted samples (in triplicate). Taking dilution into account, calculate the concentration of sucrose in the original beverage.