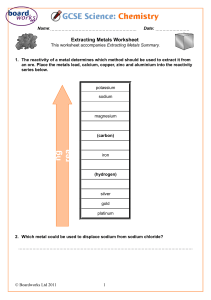
NAME: _______________________ DATE: ___________________ - State the reactions of group 1 metals with water: Record what CHANGES that you SEE happen, in the table below. METAL SYMBOL OBSERVATIONS 1. lithium 2. 3. 1. sodium 2. 3. 1. potassium 2. 3. Q Use your observations to put the metals in order of their reactivity. HIGHEST LOWEST 1. ___________________ 2. ___________________ 3. ___________________ -Describe the reaction of group 1 metals with water + complete word equations. Q Why did you put them in this order? _________________________________________ _______________________________________________________________________ Q What gas was produced? ______________. How do you know? ____________________ Q What colour did the Universal Indicator change? _______________________________ Q What does this tell you about the type of solution made? _________________________ Q What was different about the reaction of potassium? ____________________________ Q What is the pattern between the reactivity of the metals and its Group position? _______________________________________________________________________ (ASK IF YOU NEED A CLUE!) Q How are the Group 1 metals different to typical metals? 1. _____________________________________ 2. _____________________________________ NAME: ____________________________ Q Can you write/make a word equation for each reaction? 1. ____________ + ___________ __________________ + ________________ 2. ____________ + ___________ __________________ + ________________ 3. ____________ + ___________ __________________ + ________________ - Explain the reactivity of group 1 metals – including word and symbol equations Q Write a symbol equation for each reaction 1. ____________ + ___________ __________________ + ________________ 2. ____________ + ___________ __________________ + ________________ 3. ____________ + ___________ __________________ + ________________ Q Predict the reactivity of Rubidium, Caesium + Francium ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ Q Why do you think the reactivity increases as you go DOWN the group? ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ KEYWORDS: alkali metal alkaline solution group hydrogen hydroxide lithium observations periodic table potassium reactive reactivity sodium