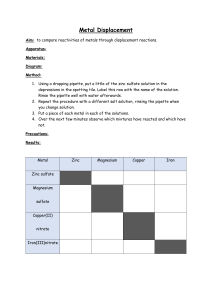
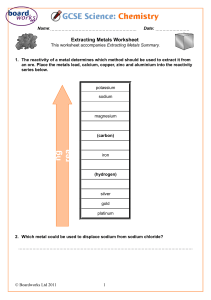
Wigmore High School Subject: Science Year group: 8 Date work set: 27th April Summer Learning 2020 Group/Set/Class: All Teacher: All Date work to be completed by: 8th May Topic: Metals and acids Learning outcomes: 1. Describe what happens when a metal reacts with an acid. 2. Use information to compare the reactivity of different metals. 3. Describe how to test for hydrogen gas. 4. Describe how metals react with oxygen and water. 5. Describe and explain what happens in displacement reactions. 6. Describe an explain how displacement reactions are used to extract metals. 7. Describe and explain the properties of ceramics and polymers. 8. Explain what composite materials are and why they are useful. The following tasks are to help you understand the content of the ‘Metals and acids’ section of our course. Please, do not attempt any of the experiments described in the activities/ websites or shown in the videos at home. These websites contain notes and videos which may be helpful in addition to the kerboodle pages listed in the activities. You can find the relevant sections by looking at the title of each activity and finding the equivalent section of the website. https://www.bbc.co.uk/bitesize/guides/zqwmxnb/revision/1 https://www.bbc.co.uk/bitesize/guides/ztxnsbk/revision/1 Metal and acid reactions Use pages 86 and 87 of the kerboodle textbook to complete this section of activities. You will need to read through the pages before completing the questions. Students set up a practical and record the following observations. 1 2 3 5 4 Each container has hydrochloric acid in it along with a metal. 1 – magnesium 2 – copper 3 – zinc 4 – iron 5 – aluminium Tasks 1. How do you know a reaction is happening in the containers? __________________________________________________ 2. What do the bubbles in the container mean is being produced in the reaction? __________________________________________________ 3. Which is the most reactive metal? How do you know? __________________________________________________ 4. Rank the containers in order or reactivity – highest first. __________________________________________________ 5. Describe one way to make sure the experiment the students completed was fair. _______________________________________________________ 6. Write a word equation for each reaction 7. Describe a test that could be used to prove that hydrogen was the gas being produced. Extension part 1: The students have an unknown metal; explain what they could do to find out how reactive a metal is (make sure you include details of fair testing). Extension part 2: The students carry out more reactions; complete the word equations. a) Lithium + nitric acid b) Sodium + sulphuric acid c) + hydrogen + lead chloride Metal and oxygen reactions Please use pages 88 and 89 of the kerboodle textbook to complete this section of activities. You will need to read through the pages first. 1. Magnesium reacts with oxygen to make magnesium oxide. a. What are the two reactants? Key words: • Reactant …………………………………………………… b. What is the product? • Product …………………………………………………… c. Write a word equation for this reaction. ………………………………………………………………………………………………… • Word equation • Chemical change 2. Which is the most reactive metal? Iron, magnesium or copper. Why do you think this? Use the table on page 89 to help you. ………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………… Extension: 3. Complete the equations for the following reactions. a. copper + oxygen → …………………………………………….. b. lithium + oxygen → ……………………………………………. c. sodium + ……………………………………… → sodium oxide d. aluminium + ……………………………….. → aluminium oxide e. …………………………….. + oxygen → potassium oxide f. …………………………….. + oxygen → calcium oxide Metal and water reactions Please use pages 90-91 of the kerboodle textbook to complete this section of activities. You will need to read through the pages before completing the activity. Task 1: Write a description of the reaction between calcium and water using page 90 of the kerboodle textbook. Task 2: Write a description of the reaction between magnesium and steam using page 91 of the kerboodle textbook. Task 3: Write down the ‘reactivity series’ of metals. This tells you the order of reactivity of metals. Reactivity is a measure of how reactive different elements are. Extension: Alkali metal + water → metal hydroxide + hydrogen Knowing this what must be the products when… Lithium + water → ___________ + _______ Sodium + water → ___________ + _______ Potassium + water → ___________ + _______ Rubidium + water → ___________ + _______ Caesium + water → ___________ + _______ Metal displacement reactions Please use pages 92 and 93 of the kerboodle textbook in order to complete this section of your work. You will need to read through the page before completing the questions. Task 1: What happens in a displacement reaction? Describe an example and explain why this is a displacement reaction. (Use the ideas about reactivity in your answer.) Task 2: The table shows the observations made when four metals are added to cold water and to dilute hydrochloric acid. (a) Write the names of these four metals in the order of their reactivity. most reactive .................................................. .................................................. ................................................. Least reactive .................................................. 1 mark (b) (i) Give the name of another metal, not in the table, which reacts in a similar way to potassium. ............................................................................................................. 1 mark (ii) What gas is formed when zinc reacts with dilute hydrochloric acid? ............................................................................................................. 1 mark (iii) The experiment with potassium and dilute hydrochloric acid should not be done in school laboratories. Suggest why it is dangerous. ............................................................................................................. ............................................................................................................. 1 mark (c) A scientist set up two test-tubes as shown below. In test-tube B the zinc strip was slowly covered with a grey deposit. Nothing happened in the other test-tube. (i) What was the grey deposit in test-tube B? ............................................................................................................. 1 mark (ii) Why was this grey deposit formed in test-tube B? ............................................................................................................. 1 mark (iii) Explain why no reaction took place in test-tube A. ............................................................................................................. ............................................................................................................. 1 mark Maximum 7 marks Extension question: Harry mixed zinc with copper sulphate solution in a test-tube. A displacement reaction took place and the temperature increased. (a) The word equation for the reaction is shown below. zinc + copper sulphate → zinc sulphate + copper Why is this reaction called a displacement reaction? ....................................................................................................................... ....................................................................................................................... 1 mark (b) Harry repeated the experiment with two other metals. He wanted to calculate the temperature rise each time. His results are shown below. metal added to copper sulphate temperature at the start (°C) highest temperature reached (°C) rise in temperature (°C) zinc 20.0 36.5 16.5 iron 25.5 38.5 13.0 magnesium 19.5 87.5 68.0 Harry used different starting temperatures. Explain why this did not affect his results. ....................................................................................................................... ....................................................................................................................... 1 mark (c) Part of the reactivity series of metals is shown below. most reactive least reactive sodium calcium magnesium aluminum zinc iron lead copper Use the reactivity series above to answer all the questions below. (i) Why was the highest rise in temperature obtained with magnesium and copper sulphate? .....................................................................................................1mark (ii) Why was the rise in temperature obtained with zinc and copper sulphate not much higher than the rise in temperature obtained with iron and copper sulphate? ................................................................................................................ ................................................................................................................ 1 mark (iii) In which of the following mixtures would there be a rise in temperature? Write yes or no in each blank box. mixture Would there be a rise in temperature? aluminum + sodium chloride calcium + zinc sulphate lead + zinc chloride magnesium + iron chloride 2 marks maximum 6 marks Extracting metals Use pages 94 and 95 of the kerboodle textbook to complete this section of the work. You will need to read through the textbook before completing the tasks. Task 1: Answer these questions using page 94 of the kerboodle textbook. 1. What is an ‘ore’? 2. What does the word ‘extract’ mean? (In the context of page 94). 3. State the two stages involved in extracting metals from their ores. Task 2: Read through the experiment below and answer the questions. 1. Write down the letters for each step in order of how the experiment should be carried out. ________________________________________________ 2. Why do you think that the carbon displaces the iron from iron oxide? _______________________________________________ 3. Which metals can be displaced from their ore using carbon? _________________________________________ 4. Which metals cannot be displaced from their ore using carbon? _________________________________________ Ceramics, polymers and composites Please use pages 96-101 to complete this section of the work. Describe what ceramics are: Give two examples: Fill in some advantages and disadvantages of using ceramics. Extension: Using your scientific knowledge, explain why this vase has a high melting point and is a hard material. In this activity, you have to use this box to fill in as much information about polymers. Make sure you include: A description of what polymers are and an example of a polymer. Extension: Add a short explanation of how monomers join together to produce polymers. Composites 1. What is a composite material? 2. Why are they made? 3. Give 2 examples of composite materials. Extension: What properties do you think the following polymers will need to have? Note: The term ‘properties’ means characteristics. For example: strong, flexible, nonbiodegradable.