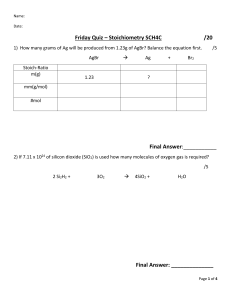
Limiting Reagent Virtual Lab Introduction: In many reactions, one reactant becomes entirely consumed and the other reactant is left over. The reactant that becomes consumed is called the limiting reagent. When the limiting reagent is all consumed, no more product can be formed (reaction complete). The reactant limits the amount of product that can be formed. Procedure: - Watch the video: https://youtu.be/OiFo7xDVZWo - Fill in the Observations as you watch - Complete all post lab questions Observations: Before reaction magnesium sulfate sodium carbonate mass: mass: Product 1: Product 2: During reaction: After reaction Mass of filter paper : Mass of filter paper and precipitate: Analysis 1. Calculate the molar concentration of the two solutions. Assume 10mL. 2. Write the balanced chemical equation for the reaction done in this lab. Include states. 3. Why was it important to put the filter paper with the product in the drying oven? 4. Identify the limiting reagent of this reaction. Show all your work. 5. Determine the theoretical yield. 6. Calculate the actual yield. 7. Calculate the percentage yield 8. Suggest reasons if the percentage yield was different from the theoretical yield.