Limiting Reagents Calculations Presentation
advertisement

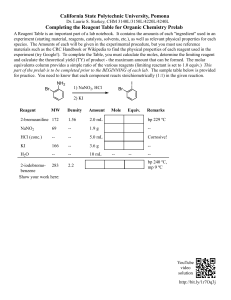
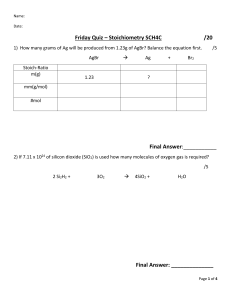
10/26/2015 Calculations Involving Limiting Reagents Learning Goals: I will be able to determine which reactant is limiting in a balanced chemical equation. I will be able to solve for amount of product produced and/or mass of product produced using stoichiometry. Limiting and Excess Reagents • Excess reagent – is the reactant that we have more than enough of. There will still be some left in the reaction vessel. • Limiting reagent – is the reactant that is completely used up in the reaction. Once we run out of it, our reaction stops. 1 10/26/2015 Applications of Limiting Reagents 1. Reduce Costs for Manufacturing – do the math, make sure that you don’t use too much of an expensive material. 2. Reduce Environmental Impact – ensure there is excess oxygen when using combustion reactions. Determining the limiting reagent. • You MUST compare the amounts using stoichiometry to see them at a 1:1 ratio…lowest number is our Limiting Reagent (L.R.) 2 10/26/2015 Example 1 – From Moles Determine the number of moles of titanium metal that can be produced when 2.8 moles of titanium(IV)chloride reacts with 5.4 mol of magnesium according the to the equation below: TiCl4(g) + Mg(l) Ti(s) + MgCl2(l) Example 2 – From Masses Aluminum hydroxide neutralizes hydrochloric acid according to the reaction below. If 0.5 g of Al(OH)3 is reacted with 0.6g of HCl, predict the mass of AlCl3 that can be produced Al(OH)3(aq) + HCl(aq) HOH(l) + AlCl3(aq) 3 10/26/2015 Today’s Tasks • Pg. 332 #1-4 • Pg. 334 #1-3 4