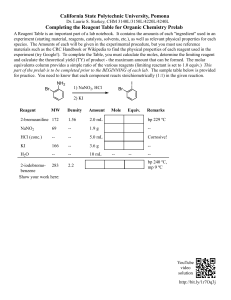
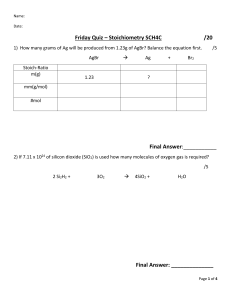
CC 102: QUALITATIVE AND QUANTITATIVE CHEMISTRY College of Liberal Arts, Sciences and Education Prepared by: ERWIN R. ABRENCILLO, PhD DOHN ARTURO M. TABERNILLA MODULE 6 STOICHIOMETRY PART III Brief Introduction or Description This module discusses the continuation of the previous topic. This focuses on the two important reagents in a chemical reaction, the limiting and excess reagents. Learning Outcomes: By the end of the module, you should be able to: 1. Differentiate limiting and excess reagents. 2. Determine the limiting reagent in a chemical reaction. 3. Solve for the excess reagent in a given chemical reaction. Lesson 1 Limiting Reagent and Excess Reagent Limiting Reagent The limiting reagent in a chemical reaction is the reactant that will be consumed completely. Once there is no more of that reactant, the reaction cannot proceed. Therefor it limits the reaction from continuing. Excess Reagent The excess reagent is the reactant that could keep reacting if the other had not been consumed. Examples 1. Let’s start with a non-chemistry example that may make more sense. • Making a car is a lot more complicated than four wheels and a steering wheel, but let's say that it’s all it takes. o You have 400 wheels and 125 steering wheels, which will you run out of first? How many of the other will be left over? • The way that it is best approached is finding out how many cars would be made based on each “reactant.” Page 1 of 3 CC 102: QUALITATIVE AND QUANTITATIVE CHEMISTRY College of Liberal Arts, Sciences and Education Prepared by: ERWIN R. ABRENCILLO, PhD DOHN ARTURO M. TABERNILLA • Because there are only enough wheels to create 100 cars, but the steering wheels can create 125 cars, the wheels are the limiting reagent. It is important to notice that the limiting reagent can be the reactant, which had more in the beginning. To find the amount of the excess (steering wheels), we need to find the amount needed to create the 100 cars. • Thus, we need 100 steering wheels to use up all the wheels. 2. Now, if we introduce chemistry back into it, the concept is the same, just a little more complex. • If we have 14.8g of propane, and 34.4g of oxygen o Determine ▪ The limiting reagent ▪ The number of moles carbon dioxide produced ▪ Mass of water produce ▪ Mass of excess reagent • First, we need a chemical equation and moles of each component. • Now that we have the amounts of propane and oxygen, we can find out how much carbon dioxide will be produced the same way we found out how many cars we had. • This means o Oxygen is the limiting reactant o .645 moles of carbon dioxide will be formed. • We can also figure out how many mols of water are produced, and therefore how much mass. • Finally, we can find the excess needed. Page 2 of 3 CC 102: QUALITATIVE AND QUANTITATIVE CHEMISTRY College of Liberal Arts, Sciences and Education Prepared by: ERWIN R. ABRENCILLO, PhD DOHN ARTURO M. TABERNILLA Learning Tasks: Answer the following problems: 1. Potassium superoxide, KO2, is used in rebreathing masks to generate oxygen according to the reaction below. If the mask contains 0.150 mol KO 2 and 0.100 mol water, how many moles of oxygen can be produced? What is the limiting reagent? 4KO2(s) + 2H2O(l) → 4KOH(s) + 3O2(g) 2. Suppose 13.7 g of C2H2 reacts with 18.5 g O2 according to the reaction below. What is the mass of CO2 produced? What is the limiting reagent? 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l) 3. Nitrogen gas can react with hydrogen gas to form gaseous ammonia. If 4.7 g of nitrogen reacts with 9.8 g of hydrogen, how much ammonia is formed? What is the limiting reagent? 4. One of the most common acids found in acid rain is sulfuric acid. Sulfuric acid is formed when gaseous sulfur dioxide reacts with ozone (O3) in the atmosphere to form gaseous sulfur trioxide and oxygen. The sulfur trioxide forms sulfuric acid when it comes in contact with water. If 5.13 g of sulfur dioxide reacts with 6.18 g of ozone, how much sulfur trioxide is formed? What is the limiting reagent? 5. Another way that sulfuric acid is formed in the atmosphere is when sulfur dioxide reacts with oxygen in a reaction catalyzed by dust in the atmosphere to form sulfur trioxide. If 7.99 g of sulfur dioxide reacts with 2.18 g of oxygen, how much sulfur trioxide can form? What is the limiting reagent? 6. In the reaction in problem #5 above, how much of the excess reactant remains after all of the limiting reactant has reacted? 7. Heating together the solids NH4Cl and Ca(OH)2 can generate ammonia. Aqueous CaCl2 and liquid H2O are also formed. If a mixture of 33.0 g each of NH 4Cl and Ca(OH)2 is heated, how many grams of NH3 will form? What is the limiting reagent? Which reactant remains in excess, and in what mass? 8. Nitrogen monoxide is formed primarily in car engines, and it can react with oxygen to form gaseous nitrogen dioxide. Nitrogen dioxide forms nitric acid when it comes in contact with water, another component of acid rain. If 3.13 g of nitrogen monoxide reacts with 4.16 g of oxygen, how much nitrogen dioxide will form? What is the limiting reagent? Which reactant remains in excess, and in what mass? References: Harvey, D V. (2010). Modern analytical chemistry. De Pauw University West, D.M. (2015) Fundamentals of analytical chemistry 9th Edition Page 3 of 3