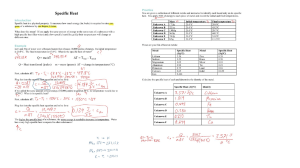
Energy changes in reactions Exothermic reaction: results in an increase in temp of the surroundings; as it gives out heat energy. Endothermic reaction: results in a decrease in temp of the surroundings; as it takes in energy. Activation energy (Ea): the minimum energy colliding particles must have to react.( energy needed to start breaking bonds) Enthalpy(H) The reaction in which the changes are happening is known as the system. Everything else is known as the surroundings. Enthalpy is the energy content of a system at constant pressure. The energy content of the reactant (H reactant) The energy content of the product (H product) = H products – H reactant Exothermic reactions The products have lower energy than the reactants. So negative. H is H is –ve because some energy left the system [ into the surrounding]. Since energy is given out into the surroundings the surrounding temp. rises. Endothermic reactions The products have more energy than the reactants. so positive. is +ve because energy was absorbed into the system [from the surroundings]. Since energy is absorbed in from the surroundings the surrounding temp decreases. is Bonds Bonds are forces of attraction between atoms/ions. Breaking these bonds requires energy; to pull the atoms apart. This energy is taken in from the surroundings. So [ temp of surroundings decreases] it's an endothermic process. Making bonds releases energy. So [ temp of surroundings increases] it's an exothermic process. Bond energy is the amount of energy ( in KJ) associated with breaking/making 1 mole of bonds. Energy difference energy required to _ energy released when break bonds bonds are made The change in energy going from reactants to product is called change in enthalpy( ) or heat of reaction. Exothermic: energy absorbed to break bonds is less than the energy released when new bonds are formed. is negative. Endothermic: energy absorbed to break bonds is more than the energy released when new bonds are formed. is positive. Calculations Ex) Measuring enthalpy change for reactions experimentally • Measuring enthalpies of combustion • Measuring enthalpies involving solutions Measuring enthalpies of combustion using calorimetry Plan an experiment to find out which fuel ethanol or propane produces more heat: • Put 100cm into the calorimeter and close it. • measure temp of water • weigh the spirt burner containing ethanol • light the burner. Stir the water with the thermometer while continuously. • put out the flame, when temp rise is about to reach 20c • weigh burner imediately • repeat previous steps using a burner containing propane. • calculate temp rise per gram of fuel burnt for each fuel. And compare. When is this method used? 1- Comparing diffrent fuels to see which would give the most heat to warm a known mass of water. 2- Compare the heat produced by the same mass of diffrent fuels How to make it a fair test? Distance between wick and can is consistent same volume of water used for all fuels Measuring enthalpies involving solutions Heat of nuetralization Polystyrene cup is a good heat insulator. It is used as simple calorimeter to measure the temperature rise of exothermic reactions between solutions. This is the procedure of the experiment • The solutions are added into the cup and initial temp is measured imediatly. • The mixture is stirred well with a thermometer. • Temp is constantly checked and maximum temperature is recorded Note: the equipment is used to measure heat energy given out during the nuetralization reactions between acids and alkalis. This energy change is known as Heat of nuetraliztion This method is suitable in cases that include: • Solid base reacting with acid • Solid carbonate reacting with acid • displacment reactions between metal ( Ch.9) Fuels A fuel is any substance we use to provide energy. We convert the chemical energy in fuels to other forms of energy. Hydrogen Fuel cell A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gais electrons at the other. At the anode: At the cathode: Overall reaction: Oxygen + Hydrogen –> Water The hydrogen-oxygen fuel cell produces energy by combing both elements.Releasing only water and energy. Summary of reaction in cell • Air entering provides the oxygen • Fuel (Hydrogen) • Hyrogen loses electrons at the anode • Oxygen reacts with the hydrogen • Water is the only product at the cathode