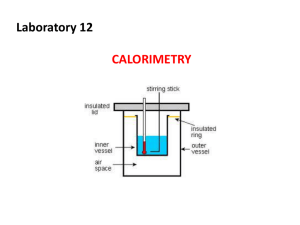
Unit 3: Thermochemistry What is Cold? https://www.youtube.com/watch?v=Akd7MMRKDwc Basic Definitions Thermochemistry: the study of energy changes that accompany physical or chemical changes of matter. Thermal Energy: the energy available from a substance as a result of the motion of its molecules. Basic Definitions System: any sample under observation (could be the coffee in a coffee cup) Chemical System: the set of reactants and products that are being studied (usually represented by a chemical equation) Surroundings: all matter around the system being studied that is capable of absorbing or releasing energy. Types of Systems OPEN SYSTEM: matter energy A chemical system in which energy and matter can move between the system and its surroundings Types of Systems CLOSED SYSTEM: One in which matter can not move in or out but energy may. y g r e en matter Types of Systems ISOLATED SYSTEM: A thermos is almost an isolated system but it does lose or gain heat over time…. energy matter A system in which neither mass or energy can move in or out; this would be the ideal way to measure energy changes in a chemical system. Measuring Energy Changes Calorimetry is the process through which energy changes in a chemical system are measured. The fancy calorimeter The Bennies science lab version Calorimeters consist of 3 main parts: •A well-insulated reaction chamber •Tight-fitting cover with insulated holes for a thermometer •Some mechanism to stir contents. When analyzing data from a coffee-cup calorimeter, assume 3 things: •Any thermal E transferred from calorimeter to the outside environment is negligible. •Any thermal E absorbed by the calorimeter itself is negligible. •All dilute, aqueous solutions have the same density (1.0g/ml) and specific heat capacity of water (4.18 j/g·˚C) Measuring Energy Changes Hard to measure thermal energy changes but you can measure: •changes in T, Volume of or mass contained in the system •pressure of system on surroundings and vice-versa Temperature – the measure of the average kinetic energy of all the particles of a sample of matter. Factors Affecting Energy Changes The factors that affect energy change in a chemical system are: •q = quantity of heat transferred - in Joules (J) •m = mass (in g) •c = specific heat capacity of a substance. •∆ T = temperature change (∆T in ˚C) Specific heat capacity The amount of energy required to raise the T of 1 gram of a substance by 1C. (units = J/g·°C) See pg. 799 for some Pure Substances. Factors affecting energy changes Heat change in a chemical system (enthalpy) can be calculated using the formula: q=mc∆T ∆T – the change in T from the beginning to the end (Tfinal – Tinitial) Endothermic vs Exothermic Energy changes are classified into endothermic or exothermic based on how energy flows between the chemical system and its surroundings. •If energy is lost to the surroundings, the change is EXOTHERMIC. •q is negative for the system: System transfers thermal energy to surroundings. Endothermic vs Exothermic •If energy is absorbed from its surroundings, the change is ENDOTHERMIC. •q is positive for the system • System absorbs thermal energy from surroundings. More on this tomorrow… Enthalpy Change •symbol: ΔH •the difference in enthalpies of reactants and products during a change •determined from the energy changes of the surroundings For a given reaction, the enthalpy change, ΔH, is given by: ΔH = Hproducts - Hreactants Examples and Communication 1. A student has 125.0 mL of water at 20.0˚C, then places a sample of silver at 125˚C into the calorimeter. The final temperature of the water is 22.5 ˚C. Calculate the quantity of thermal energy absorbed by the water. 2. What quantity of energy is transferred to cool 25.0g of water from 100.0˚C to 20.0˚C?