Endothermic and Exothermic Reaction
“When trying to classify a reaction as exothermic or endothermic, watch
how the temperature of the surrounding—in this case, the flask—
changes. An exothermic process releases heat, causing the temperature
of the immediate surroundings to rise. An endothermic process absorbs
heat and cools the surroundings.”
Based on the above definition, let's pick a few examples from our daily
lives and categorize them as endothermic or exothermic.
Endothermic reactions: Heat is absorbed.
1) Photosynthesis: Plants absorb heat energy from sunlight to convert
carbon dioxide and water into glucose and oxygen.
6CO2 + 6 H2O + heat ---> C6H12O6 + 6O2
2) Cooking an egg: Heat energy is absorbed from the pan to cook the
egg.
Exothermic reactions: Heat is released.
1) Combustion: The burning of carbon-containing compounds uses
oxygen, from air, and produces carbon dioxide, water, and lots of heat.
For example, combustion of methane (\text{CH}_4CH4start text, C, H,
end text, start subscript, 4, end subscript) can be represented as follows:
CH4 + 2(O2) ---> CO2 + 2H2O + heat
2) Rain: Condensation of water vapor into rain releasing energy in the
form of heat is an example of an exothermic process.
Why is heat released or absorbed in a chemical
reaction?
In any chemical reaction, chemical bonds are either broken or formed.
And the rule of thumb is "When chemical bonds are formed, heat is
released, and when chemical bonds are broken, heat is absorbed."
Molecules inherently want to stay together, so the formation of chemical
bonds between molecules requires less energy as compared to breaking
bonds between molecules, which requires more energy and results in
heat being absorbed from the surroundings.
What is the enthalpy of a reaction?
Enthalpy of a reaction is defined as the heat energy change (ΔHΔHΔ,
H) that takes place when reactants go to products. If heat
is absorbed during the reaction, ΔHΔHΔ, H is positive; if heat is released,
then ΔHΔHΔ, H is negative.
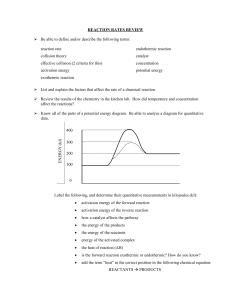
Depiction of an energy diagram
In a chemical reaction, some bonds are broken and some bonds are
formed. During the course of the reaction, there exists an intermediate
stage, where chemical bonds are partially broken and partially formed.
This intermediate exists at a higher energy level than the starting
reactants; it is very unstable and is referred to as the transition state. The
energy required to reach this transition state is called activation energy.
We can define activation energy as the minimum amount of energy
required to initiate a reaction,
An energy diagram can be defined as a diagram showing the relative
potential energies of reactants, transition states, and products as a
reaction progresses with time.
Let’s draw an energy diagram for the following reaction:
Activation energy graph for CO (g) + NO2 (g) ---> CO2 (g) + NO (g)
The activation energy is the difference in the energy between the
transition state and the reactants. It’s depicted with a red arrow.
Energy diagrams for endothermic and
exothermic reactions
In the case of an endothermic reaction, the reactants are at a lower
energy level compared to the products—as shown in the energy diagram
below. In other words, the products are less stable than the reactants.
Since we are forcing the reaction in the forward direction towards more
unstable entities, overall ΔHΔHΔ, H for the reaction is positive, i.e.,
energy is absorbed from the surroundings.
Image of a graph showing potential energy in relation to the process of a
chemical reaction.
In the case of an exothermic reaction, the reactants are at a higher
energy level as compared to the products, as shown below in the energy
diagram. In other words, the products are more stable than the
reactants. Overall enthalpy for the reaction is negative, i.e., energy is
released in the form of heat.
Image of a graph showing potential energy in relation to the process of
an exothermic reaction.