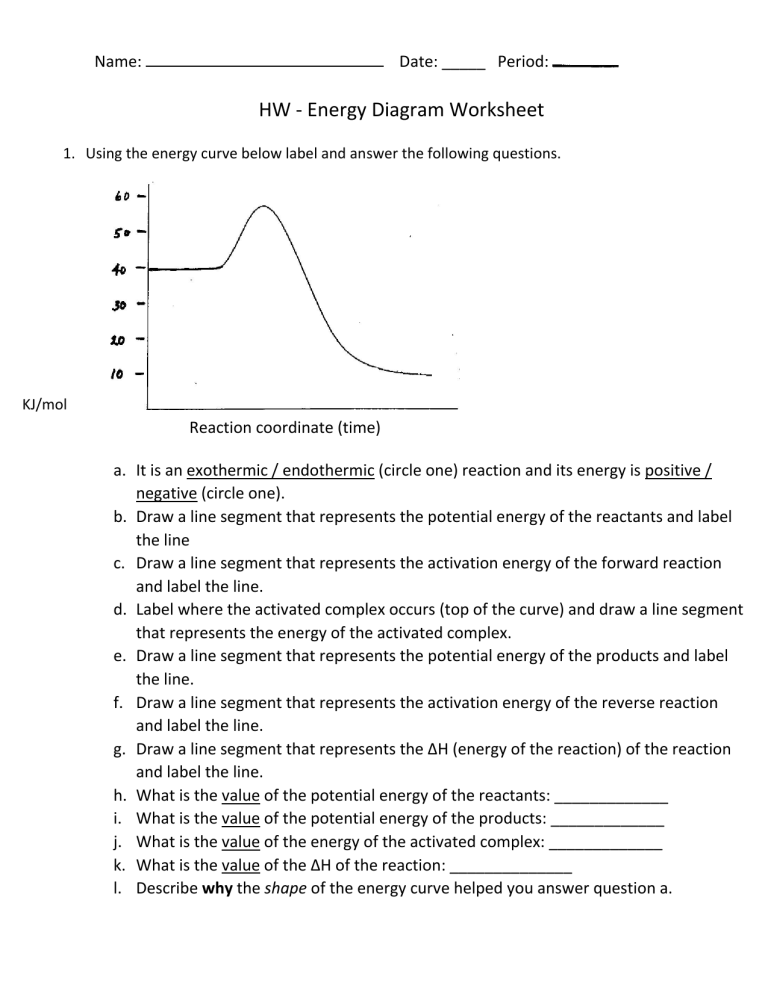
Name: Date: _____ Period: HW - Energy Diagram Worksheet 1. Using the energy curve below label and answer the following questions. KJ/mol Reaction coordinate (time) a. It is an exothermic / endothermic (circle one) reaction and its energy is positive / negative (circle one). b. Draw a line segment that represents the potential energy of the reactants and label the line c. Draw a line segment that represents the activation energy of the forward reaction and label the line. d. Label where the activated complex occurs (top of the curve) and draw a line segment that represents the energy of the activated complex. e. Draw a line segment that represents the potential energy of the products and label the line. f. Draw a line segment that represents the activation energy of the reverse reaction and label the line. g. Draw a line segment that represents the ∆H (energy of the reaction) of the reaction and label the line. h. What is the value of the potential energy of the reactants: _____________ i. What is the value of the potential energy of the products: _____________ j. What is the value of the energy of the activated complex: _____________ k. What is the value of the ∆H of the reaction: ______________ l. Describe why the shape of the energy curve helped you answer question a. Practice Regents Constructed Response Questions 2) Nitrogen gas reacts with oxygen gas to form nitrogen oxide (NO) gas according to the following chemical equation: N2(g) + 02(g) 2N0(g) (a) According to your reference table in the Heats of Reaction at 101.3 kPa and 298 K, is this reaction exothermic or endothermic. [Explain your answer.] (b) Is heat energy absorbed or released during this reaction? (c) If 1 mole of NO(g) is produced, how many kilojoules of heat energy are absorbed or released? (d) On the axis below, draw a potential energy diagram for the reaction. Label the reactants and the products on your graph. [Do not number the axis.] Reaction Coordinate (e) Draw an arrow on your diagram to represent the heat of the reaction. Label the arrow ∆H.