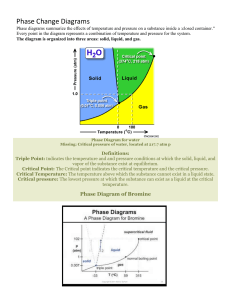
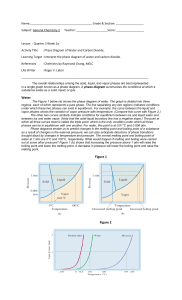
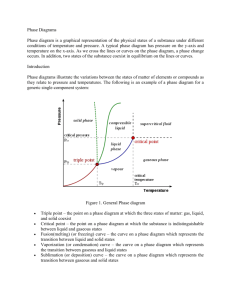
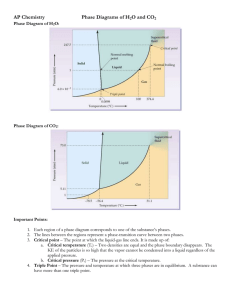
PHASE CHANGES AND PHASE DIAGRAM Phase changes are transformations of matter from one physical state to another. They occur when energy (usually in the form of heat) is added or removed from a substance. They are characterized by changes in molecular order; molecules in the solid phase have the greatest order, while those in the gas phase have the greatest randomness or disorder. Types of Phase Changes • The change from solid to liquid is melting, liquid to gas is vaporization, and solid to gas is sublimation. These changes take place when heat is absorbed (heat gained). They are endothermic processes. • The reverse change from gas to liquid is condensation, gas to solid is deposition, and liquid to solid is freezing. These changes give off heat (heat lost) and are exothermic processes. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. It gives the possible combinations of pressure and temperature at which certain physical state or states a substance would be observed. Each substance has its own phase diagram. What are the features of a phase diagram? Phase diagrams are plots of pressure (1 atm = 101.3 kpa = 760 mm Hg) versus temperature (usually in degrees Celsius or Kelvin). A. The Three Areas The three areas are marked solid, liquid, and vapor. Under a set of conditions in the diagram, a substance can exist in a solid, liquid, or vapor (gas) phase. The labels on the graph represent the stable states of a system in equilibrium. Suppose a pure substance is found at three different sets of conditions of temperature and pressure corresponding to A, B, and C as shown in the following diagram. Under the set of conditions at A in the diagram, the substance would be a solid as it falls into that area of the phase diagram. At B, it would be a liquid; and at C, it would be a vapor (gas). B. Three Lines (Curves) The lines that serve as boundaries between physical states represent the combinations of pressures and temperatures at which two phases can exist in equilibrium. In other words, these lines define phase change points. 1. The green line divides the solid and liquid phases, and represents melting (solid to liquid) and freezing (liquid to solid) points. Melting (or freezing) curve – the curve on a phase diagram which represents the transition between liquid and solid states. It shows the effect of pressure on the melting point of the solid. Anywhere on this line, there is equilibrium between the solid and the liquid. 2. The blue line divides the liquid and gas phases, and represents vaporization (liquid to gas) and condensation (gas to liquid) points. Vaporization (or condensation) curve – the curve on a phase diagram which represents the transition between gaseous and liquid states. It shows the effect of pressure on the boiling point of the liquid. Anywhere along this line, there will be equilibrium between the liquid and the vapor. 3. The red line divides the solid and gas phases, and represents sublimation (solid to gas) and deposition (gas to solid) points. Sublimation (or deposition) curve – the curve on a phase diagram which represents the transition between gaseous and solid states. It represents the effect of increased temperature on a solid at a very low constant pressure, lower than the triple point. C. Two Important Points 1. The triple point The triple point is the combination of pressure and temperature at which all three phases of matter are at equilibrium. It is the point on a phase diagram at which the three states of matter coexist. The lines that represent the conditions of solid-liquid, liquid-vapor, and solid-vapor equilibrium meet at the triple point. 2. The critical point The critical point terminates the liquid/gas phase line. The critical point, the temperature above which the gas cannot be liquefied no matter how much pressure is applied (the kinetic energy simply is too great for attractive forces to overcome). Beyond the temperature of the critical point, the merged single phase is known as a supercritical fluid (indistinguishable between gas or liquid- neither one). The temperature and pressure corresponding to this are known as the critical temperature and critical pressure. The Phase Diagram for Water Notice that the triple point for water occurs at a very low pressure, 0.006 atm and at 273.2 K temperature. Also notice that the critical temperature is 647 K (374°C). It would be impossible to convert water from a gas to a liquid by compressing it above this temperature. The critical pressure is 218 atm. The normal melting and boiling points of water are found in exactly the same way as we have already discussed - by determining where the 1 atm pressure line crosses the solid-liquid, and then the liquid-vapor equilibrium lines. The normal melting point of water is 273 K (0 oC), and its normal boiling point is 373 K (100 oC). The Phase Diagram for Carbon Dioxide The only thing special about this phase diagram is the position of the triple point, which is well above atmospheric pressure. It is impossible to get any liquid carbon dioxide at pressures less than 5.2 atmospheres. At 1 atm pressure, carbon dioxide will sublime at a temperature of 197.5 K (-75.5 °C). This is the reason why solid carbon dioxide is often known as "dry ice." There is no liquid carbon dioxide under normal conditions - only the solid or the vapor. Name: _________________________________________ STEM 12- ___ Group No: _____ Score: ___________ Date: ____________ ACTIVITY 6- INTERPRETING PHASE DIAGRAM Direction. Refer to the following phase diagram of a certain substance to answer the following questions. Part A. Part B. 1. What phases of water coexist at point C in water’s phase diagram? 1. In what phase is the substance at 50 °C and 1 atm pressure? 2. At what pressure and temperature conditions will all three phases of the substance be present? 2. What two phase changes occur at point D in the phase diagram for water? 3. What is the critical temperature of water? 3. What is the normal melting point of the substance? 4. What pressure is at water’s normal boiling point? 4. What phase(s) will exist at 1 atm and 70 °C? 5. What occurs at the triple point? Part C. Part D. 1. Look at the phase diagram for carbon dioxide. Above which pressure and temperature is carbon dioxide unable to exist as a liquid? 1. What is the temperature at which the triple point occurs? 2. At which pressure and temperature do the solid, liquid, and gaseous phases of carbon dioxide coexist? 2. What 2 phase changes occur at Point A? 3. What two phase changes occur at point E in the phase diagram for carbon dioxide? 3. What phase change does the substance at 100 bars undergo as the temperature decreases from 250 K to 200 K? 4. What phase change occurs as carbon dioxide moves from -78°C to 24°C at a pressure of 50 atm? 4. What is the pressure at which the critical point occurs? 5. What 2 phase changes occur at Point B?