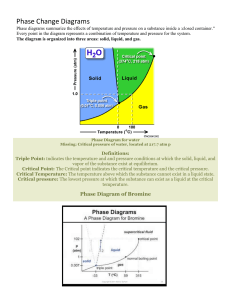
Name:______________________________________ Grade & Section: _________________ Subject: General Chemistry 2 Teacher: ___________________Score: _________________ Lesson : Quarter 3 Week 2a Activity Title : Phase Diagram of Water and Carbon Dioxide. Learning Target : Interpret the phase diagram of water and carbon dioxide. References : Chemistry by Raymond Chang, MELC LAS Writer : Roger V. Labor ______________________________________________________________________________ The overall relationships among the solid, liquid, and vapor phases are best represented in a single graph known as a phase diagram. A phase diagram summarizes the conditions at which a substance exists as a solid, liquid, or gas. Water The Figure 1 below (a) shows the phase diagram of water. The graph is divided into three regions, each of which represents a pure phase. The line separating any two regions indicates conditions under which these two phases can exist in equilibrium. For example, the curve between the liquid and vapor phases shows the variation of vapor pressure with temperature. (Compare this curve with Figure 2.) The other two curves similarly indicate conditions for equilibrium between ice and liquid water and between ice and water vapor. (Note that the solid-liquid boundary line has a negative slope.) The point at which all three curves meet is called the triple point, which is the only condition under which all three phases can be in equilibrium with one another. For water, this point is at 0.01°C and 0.006 atm. Phase diagrams enable us to predict changes in the melting point and boiling point of a substance as a result of changes in the external pressure; we can also anticipate directions of phase transitions brought about by changes in temperature and pressure. The normal melting point and boiling point of water at 1 atm are 0°C and 100°C, respectively. What would happen if melting and boiling were carried out at some other pressure? Figure 1 (b) shows that increasing the pressure above 1 atm will raise the boiling point and lower the melting point. A decrease in pressure will lower the boiling point and raise the melting point. Figure 1 Figure 2 (a) The phase diagram of water. Each solid line between two phases specifies the conditions of pressure and temperature under which the two phases can exist in equilibrium. The point at which all three phases can exist in equilibrium (0.006 atm and 0.01°C) is called the triple point. (b) This phase diagram tells us that increasing the pressure on ice lowers its melting point and that increasing the pressure of liquid water raises its boiling point. Carbon Dioxide The phase diagram of carbon dioxide (Figure 3) is generally similar to that of water, with one important exception—the slope of the curve between solid and liquid is positive. In fact, this holds true for almost all other substances. Water behaves differently because ice is less dense than liquid water. The triple point of carbon dioxide is at 5.2 atm and 257°C. An interesting observation can be made about the phase diagram in Figure 3. As you can see, the entire liquid phase lies well above atmospheric pressure; therefore, it is impossible for solid carbon dioxide to melt at 1 atm. Instead, when solid CO 2 is heated to 278°C at 1 atm, it sublimes. In fact, solid carbon dioxide is called dry ice because it looks like ice and does not melt (Figure 3). Because of this property, dry ice is useful as a refrigerant. Figure 3 The phase diagram of carbon dioxide. Note that the solid-liquid boundary line has a positive slope. The liquid phase is not stable below 5.2 atm, so that only the solid and vapour phases can exist under atmospheric conditions. Review Concepts 1. 2. For every substance there is a temperature, called the critical temperature, above which its gas phase cannot be made to liquefy. The relationships among the phases of a single substance are illustrated by a phase diagram, in which each region represents a pure phase and the boundaries between the regions show the temperatures and pressures at which the two phases are in equilibrium. At the triple point, all three phases are in equilibrium. Questions and Problems 1. 2. 3. What is a phase diagram? What useful information can be obtained from the study of a phase diagram? Explain how water’s phase diagram differs from those of most substances. What property of water causes the difference? A phase diagram of water is shown at the end of this problem. Label the regions. Predict what would happen as a result of the following changes: (a) Starting at A, we raise the temperature at constant pressure. (b) Starting at C, we lower the temperature at constant pressure. (c) Starting at B, we lower the pressure at constant temperature.