Section Summary 13.4
advertisement

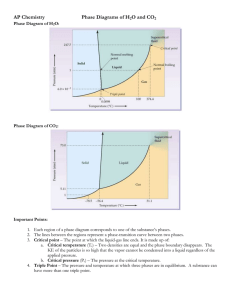
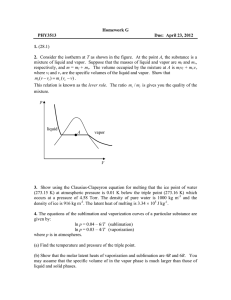
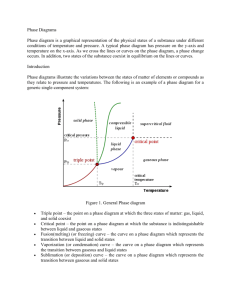
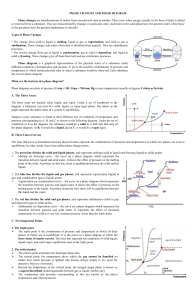
SECTION SUMMARY 13.4 Changes of State Summary: The change that occurs when a solid goes directly to the gas or vapor state without first becoming a liquid is sublimation. This change can occur because solids, like liquids, have a vapor pressure. Substances that sublime include iodine and solid carbon dioxide (dry ice). A graph that shows the relationship between the states of a substance is called a phase diagram. On this diagram, a line between two phases shows the conditions at which the phases are in equilibrium. The triple point is the only set of conditions at which solid, liquid, and gas phases coexist. The triple point for water is the temperature of 0.016 degree Celsius and a pressure of 0.61 kPa. A phase diagram does not give information on changes in mass of solids, liquids, and gases. Based on phase diagram, water can be made to boil at 1050C by increasing pressure. 13.4 Changes of State Vocabulary Terms: melting: the change of a solid to a liquid state freeze drying: a method or removing water from food, using sublimation phase diagram: graph that shows the relationship among the states of a substance 0.016oC and 0.61 kPa: temperature and pressure of the triple point of water sublimation: the change of a solid to a vapor without passing through the liquid state 100oC and 101.3 kPa: the temperature and pressure at the normal boiling point of water triple point: the only point describes the only set of conditions at which all three phases can exist in equilibrium with one another The End of the Summary