AP Chemistry Phase Diagrams of H O and CO
advertisement
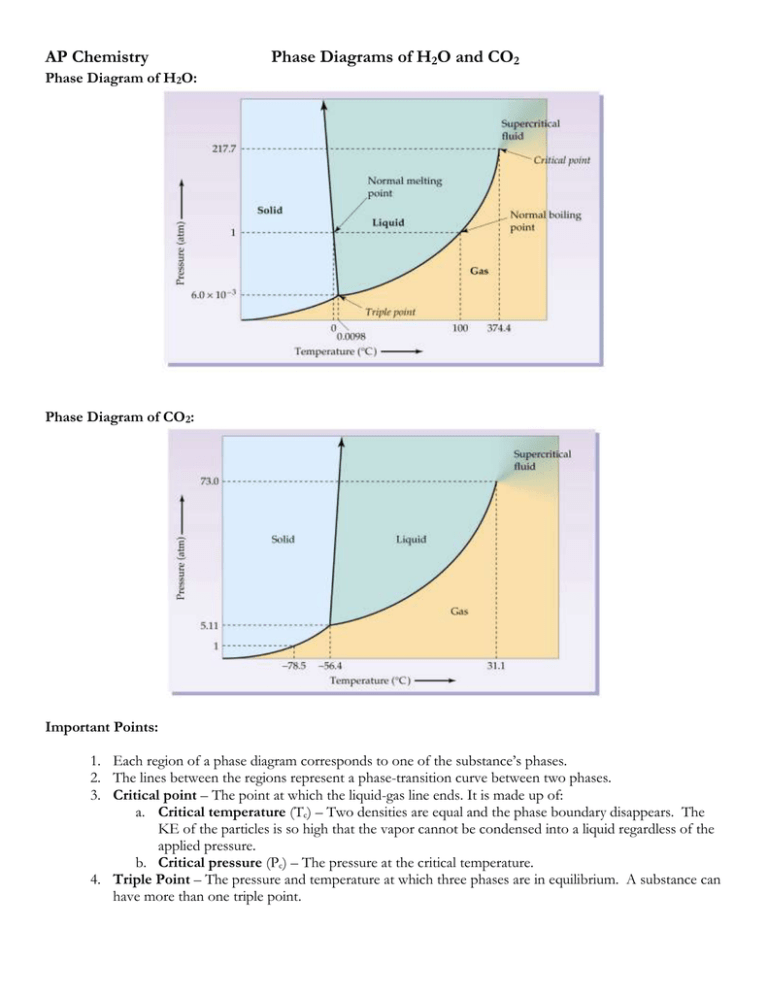
AP Chemistry Phase Diagrams of H2O and CO2 Phase Diagram of H2O: Phase Diagram of CO2: Important Points: 1. Each region of a phase diagram corresponds to one of the substance’s phases. 2. The lines between the regions represent a phase-transition curve between two phases. 3. Critical point – The point at which the liquid-gas line ends. It is made up of: a. Critical temperature (Tc) – Two densities are equal and the phase boundary disappears. The KE of the particles is so high that the vapor cannot be condensed into a liquid regardless of the applied pressure. b. Critical pressure (Pc) – The pressure at the critical temperature. 4. Triple Point – The pressure and temperature at which three phases are in equilibrium. A substance can have more than one triple point.