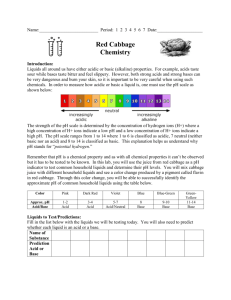
Red Cabbage Lab: Acids and Bases Introduction: Liquids all around us have either acidic or basic (alkaline) properties. For example, acids taste sour; while bases taste bitter and feel slippery. However, both strong acids and strong bases can be very dangerous and burn your skin, so it is important to be very careful when using such chemicals. In order to measure how acidic or basic a liquid is, one must use the pH scale as illustrated below: The strength of the pH scale is determined by the concentration of hydrogen ions (H+) where a high concentration of H+ ions indicate a low pH and a high concentration of OH- ions indicate a high pH. The pH scale ranges from 1 to 14 where 1 to 6 is classified as acidic, 7 is neutral, and 8 to 14 is classified as basic. In this lab, you will use the juice from red cabbage as a pH indicator to test common household liquids and determine their pH levels. You will mix cabbage juice with different household liquids and see a color change produced by a pigment called flavin in the red cabbage. Through this color change, you will be able to successfully identify the approximate pH of common household liquids using the table below: Color Pink Dark Red Violet Blue BlueGreen GreenYellow Approximate pH 1-2 3-4 5-7 8 9 – 10 11 - 12 Acid/Base Acid Acid Acid/Neutral Base Base Base Materials: 1. Small Beaker or Cup 2. Beaker or Cup of Red Cabbage Juice Indicator 3. Micropipettes 4. White Vinegar 5. Lemon Soda 6. Coca Cola 7. Bleach 8. Ammonia 9. Baking Soda 10. Shampoo Pre-Lab Predictions: Look at each of the liquids being tested. Predict whether each of the substances is acidic, neutral, or basic. Circle one. White Vinegar Acidic Neutral Basic Lemon Soda Acidic Neutral Basic Coca Cola Acidic Neutral Basic Bleach Acidic Neutral Basic Ammonia Acidic Neutral Basic Baking Soda Acidic Neutral Basic Shampoo Acidic Neutral Basic Procedure: 1. Use a sharpie to label each of the Dixie Cups with the liquid that it will contain. 2. Send one member of your lab group to the supplies table with the labeled Dixie cups. Add 2 pipettes of the solution to its corresponding cup. This may take more than one trip! 3. Once all of the cups are back at your lab table. Add one pipette of red cabbage juice to the cups one by one. Observe and record any color change in the data table below. Data: Table 1: pH of Household Solutions Household Solution Color Change Predicted pH Actual pH White Vinegar 5.0 Lemon Soda 3.4 Coca Cola 2.8 Bleach 12.0 Ammonia 11.6 Baking Soda 10.0 Shampoo 6.5 Table 2: Categorize your results below Strong Acids Weak Acids Neutral Weak Bases Strong Bases Follow-Up Questions: 1.) Were the pH’s predicted by the red cabbage juice indicator close to the actual pH of the solution? How accurate is red cabbage juice as an acid/base indicator? 2.) Of the solutions tested, which solution would have the greatest concentration of hydrogen ions? What about hydroxide ions? 3.) What defines an acid, base, or neutral substance? 4.) Whenever you mix an acid with a base, they neutralize each other. If this is the case, why is Alka-Seltzer used to treat stomach aches? (Note: excess stomach acids cause stomach aches) 5.) What is acid rain and how is it a problem to oceans, rivers, lakes, ponds, etc.? 6.) Knowing what you know about acid rain and acid/base chemistry, think of and describe a working scientific solution to the corrosion caused by acid rain.