ction 2-3 Solutions
advertisement

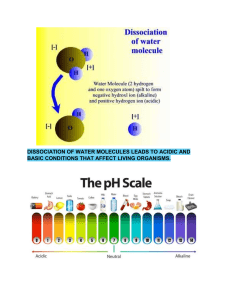
ction 2-3 Solutions 54. Many of the chemical reactions in organisms take place in __________. 55. What is a solution? 56. Give an example of a complex solution in your body. 57. Name & describe the 2 parts of a solution. 58. What is meant by concentration of the solution? 59. How do you get a saturated solution? 60. What are aqueous solutions? 61. Explain dissociation of water molecules. 62. Name and give the charge for the 2 ions formed whenever water dissociates. 63. Write the final equation for the dissociation of water. 64. What is the hydronium ion? 65. How are acidity and alkalinity measured? 66. When would a solution be neutral? Give an example of a neutral solution. 67. When would solutions be considered as acidic? 68. Acids have what taste? 69. Acids form what ion in water? 70. Give an example of an acid in your stomach. 71. When would solutions be considered as a base? 72. What adjective refers to basic solutions? 73. Give an example of a base. 74. What ion forms whenever a base is dissolved in water? 75. How does a base taste and feel? 76. How is soap made? 77. What is the pH scale used for? 78. What is the range for the pH scale? 79. At what pH would you find each of these solutions on a pH scale: Bases? c. neutral? a. acids? 80. How many times stronger is a pH of 3 than a pH of 5? 81. A change of one pH unit reflects a __________ change. 82. Why is controlling the pH range important to organisms? 83. How do organisms control their pH levels? 84. What is a buffer? 85. Give an example of a human body fluid that is: a. acidic? b. alkaline? b.