pH Lab
advertisement

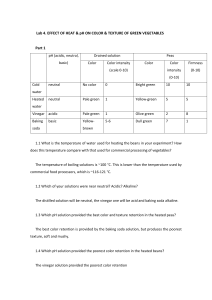
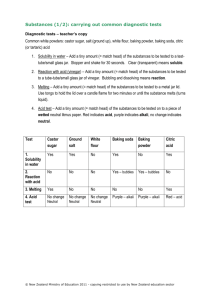
Name: pH Lab The pH scale is used to measure the strength of an __________ or a _______________. The pH scale runs from ____ to ____. ________- a substance with a pH _____________ than 7.0 -The __________ the pH, the more acidic the solution ____________- a solution in which the pH is ____________ to 7.0 -Water is an example of a neutral solution ______________- have a pH that is ________________ than 7.0 -The ________ the pH, the more basic (or alkaline) the solution pH scale: Neutral -------------------------------------------------------------------- Strong Weak Weak Strong acid acid base base __________help maintain homeostasis by minimizing changes in pH. pH of Common Substances Lab Pre-Lab Questions: 1. What does pH measure? 2. What is the pH value of a neutral solution? Give an example! 3. Is the pH of a strong base higher or lower than the pH of a weak base? 4. Is the pH of a strong acid higher or lower than the pH of a weak acid? Name: Procedure: 1. Pour small amounts of each substance to be tested into an appropriately labeled cup 2. Following the directions for the pH strips, take a pH reading of each substance and record it in the table. Determine whether the substance is an acid or a base. 3. Compare your results to your predictions and to the results of the rest of the class. Hypotheses: Form hypotheses stating whether you think each substance will be an acid, a base, or neutral. a. Lemon Juice: b. Windex: c. Dish soap: d. Baking soda/water: e. Tap water: f. vinegar g. Bleach h. egg white Data Table Substance Predicted pH Predicted acid or base? Actual pH Actual acid or base? Lemon Juice Windex Dish Soap Baking soda/water Tap water Vinegar Bleach Egg White Using the data from Table 1, add labels showing the pH of each test substance to the pH scale below. 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Acids Bases Results/Conclusion Questions: 1. How did your predictions compare with your results? Why did you think certain substances would be acidic or basic? 2. Which substance was the strongest acid? ________________ Weakest base? ____________ 3. In living things, pH is maintained within a narrow range because it is vital for homeostasis.” This means …