22 The Learning Station Science Grade 8 PRe
advertisement

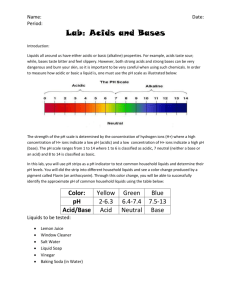
The Learning Station 22 Science Grade 8 PRE-LAB FOR UNKNOWN SOLUTIONS LAB (MONDAY, DEC. 17TH, 19TH) NOTICE: Please note that this document is to be studied for the lab, you will not have access to it during the lab nor will you have access to your textbook. Please read carefully. OBJECTIVE: to determine the identity of nine unknown solutions which may be acids, bases or of neutral nature. To do this you will conduct multiple tests. THEORY TO REVIEW BEFORE COMING TO THE LAB: 1. P. 183 to188: please go over table 4, p.184. The dangerous of acids and bases. 2. Concentrations of acids and bases 3. Neutralisation: a reaction that occurs between an acid and a base. When two equally concentrated solutions of an acid and a base meet, the react by neutralising one-another and rendering the final combination of these two solutions neutral. 4. pH scale: power of 10 between each value. 5. Magnesium ribbon test: a small ribbon of magnesium metal is used to test if a solution is of acidic nature. If it is acidic, there will be a reaction in which the ribbon bubbles to release hydrogen gas. What is leftover is a solution of magnesium salt, the solution is neutral. The magnesium ribbon test does nothing to basic or neutral solutions. 6. Phenolphthalein test: Phenolphthalein is a clear indicator solution used to test if a solution is of basic nature. If the solution is indeed basic, adding drops of phenolphthalein will turn it pink. This indicator does nothing to acids or neutral solutions. 7. pH meter: an electrical instrument to test whether a solution is of acidic, basic or salt nature. It does nothing for neutral solutions. It does so by beeping or lighting an LED light when in contact with an acidic, basic or salty solution. 8. Universal pH paper: use small pieces of this paper to dip into the nine solutions. Wait one minute. This paper will then change into one of 14 colours that represent the pH scale. Use the chart that comes with the container to locate the colour and what pH value it represents. 9. What is acid rain: acid rain is rain of more acidic nature due to pollutions releasing contaminants that interact with water rendering it more acidic. 1