Flame Tests: Identifying Metal Ions in Chemistry
advertisement

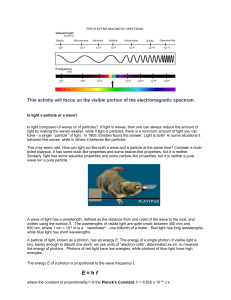
Flame tests Flame tests are used in chemistry to identify the metal ions in compounds. A small amount of the compound being tested will be held in a flame and the colour given off observed. The colour is caused by the movement of electrons in the metal ion. When heated, the electrons gain energy, and ‘jump’ up an energy level; however, they quickly fall back down to their original energy levels, releasing the extra energy as light as they do so. The arrangement of the particular elements electron shells determines the wavelength of light released and gives the characteristic flame colours of different metal ions. Compound Strontium Chloride Barium Chloride Calcium chloride Sodium chloride Potassium chloride Copper chloride Colour