Flame Test Lab Report: Identifying Elements by Color
advertisement

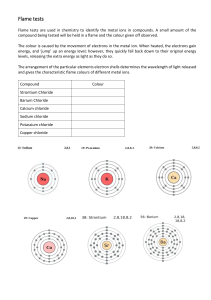
The Flame Test Problem: To repeat the flame test that was done by Neils Bohr. - Study fig 11 pg 232 and re-read what Neils Bohr did Materials: Bunsen burner, flask, wooden splints, lighter and chemical solutions Procedure: 1. At a lab bench, set up a bunsen burner and light it. 2. Place the soaked end of the wooden splint in the hottest part of the flame for 5 seconds. 3. Observe the colour which appears. 4. Go to the next station. Observations Compound Name 1 2 3 4 5 6 7 8 9 10 Flame Colour Element Present Observations Compound Name Flame Colour Element Present 1 Copper sulphate green Copper 2 Potassium iodide violet Potassium 3 Copper (II) chloride green Copper 4 Calcium Chloride Red-orange Calcium 5 Strontium Chloride Crimson red Strontium 6 Lithium Chloride Red Lithium 7 Potassium Chloride violet Potassium 8 Barium Chloride Yellow-green Barium 9 Sodium Chloride Orange-yellow Sodium 10 Potassium Carbonate violet Potassium Discussion 1. How could you determine if the element copper was in a certain compound? 2. Based on what you have learned, what colour do you think these solutions would produce: A: Lithium bromide B: Barium Sulfate 3. Burning elements to produce different colours has one commercial use. Can you think of where you may have seen this in action? Conclusion Based on the reading on pg 232, write a brief conclusion about what is happening to the electrons as the element is heated and what we learned in this lab.