Flame Test Lab Worksheet: Element Identification & Fireworks
advertisement

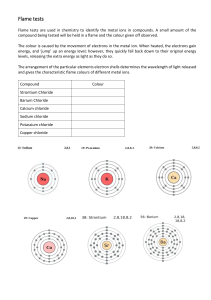
Name__________________________________________ Date ________________ Period _________ Flame Test Lab Purpose:_____________________________________________________________________________ _____________________________________________________________________________________ Hypothesis:___________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ Materials:____________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ Procedure: Data Table: Printed on Reverse side of this paper Questions to Answer: 1.) Why is it necessary to clean the wire loop between flame tests? 2.) What colors would you see if your teacher gave you a sample of Potassium Chloride? Of Strontium Chloride? Of Copper Chloride? 3.) You have just returned from the moon with a handful of moon rocks. Describe how you might identify some of the basic metals in the moon rock. 4.) A pan of milk boils over on the stove at home. The flame turns orange-red. Explain why it turns orange-red 5.) During a fireworks display you see a bright red flash. What element might be used in the fireworks display? 6.) What were the unknowns? #1 _________________ #2 ________________ #3 ________________ 1.) Element Name (symbol) Potassium (K) 2.) Sodium (Na) 3.) Strontium (Sr) 4.) Lithium (Li) 5.) Copper (Cu) 6.) Calcium (Ca) 7.) Cobalt (Co) 8.) Iron (Fe) 9.) Tin (Sn) 10.) Unknown #1 11.) Unknown #2 12.) Unknown #3 Flame color