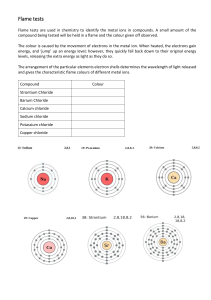
Demonstration: Flame Tests (Rainbow Demo) FOR THE TEACHER Summary In this demonstration, students will observe the variety of colors produced when different metals or metallic salts are heated in a flame. Created by AACT Grade Level High School NGSS Alignment This activity will help prepare your students to meet the performance expectations in the following standards: HS-PS4-1: Use mathematical representations to describe a simple model for waves that includes how the amplitude of a wave is related to the energy in a wave. HS-PS4-3: Evaluate the claims, evidence, and reasoning behind the idea that electromagnetic radiation can be described either by a wave model or a particle model, and that for some situations one model is more useful than the other. Scientific and Engineering Practices: o Analyzing and Interpreting Data Objectives By the end of this demonstration, students should be able to Use flame tests to identify a metal or metallic salt by the color that it produces when it is put into a flame. Calculate the frequency of light given its wavelength. Calculate the wavelength of light given its frequency. Identify an unknown metal by the color it emits when passed through a flame. Chemistry Topics This lesson supports students’ understanding of Energy Electromagnetic Spectrum Atomic structure Atomic spectra Electrons Visible light spectrum Time Teacher Preparation: 30 minutes, longer if solution preparation is necessary Lesson: 45 minutes Materials 7, 100 mL beakers Wooden splints (number depends upon how many times the demo will be performed) 1 Bunsen burner 1 Striker 50 mL of 1.0M Barium Chloride (BaCl2) – Note: Barium chloride is highly toxic. Do not ingest the salt or solution. 50 mL of 1.0M Calcium Chloride (CaCl2) 50 mL of 1.0M Copper Chloride (CuCl2) 50 mL of 1.0M Lithium Chloride (LiCl) American Association of Chemistry Teachers |1-395 1 50 mL of 1.0M Potassium Chloride (KCl) 50 mL of 1.0M Sodium Chloride (NaCl) 50 mL of 1.0M Strontium Chloride (SrCl2) Deionized water 250 mL beaker or plastic or cardboard cup half full of water Class copies of the visible light spectrum chart showing wavelength and frequency values Example Safety Barium chloride is highly toxic. Do not ingest the salt or solution. Students should wear proper safety gear during chemistry demonstrations. Safety goggles and lab apron are required. Wear proper personal protective equipment when preparing and working with solutions. Always use caution around open flames. Keep flames away from flammable substances. Always be aware of an open flame. Open flames can cause burns. Do not reach over it, tie back hair, and secure loose clothing. Wash hands after handling materials used to prepare for or perform this experiment. Teacher Notes Additional safety information: The Rainbow Demonstration (or Rainbow Flame Demonstration) is an activity popularly conducted in chemistry classrooms. The purpose is to demonstrate to students the variety of colors produced when different metals or salts meet a flame. This procedure is an updated version of the traditional one. The traditional Rainbow Demonstration required that a highly flammable solvent, such as methanol or ethanol, be mixed with the metallic salts. The American Chemical Society Committee on Chemical Safety recommends that this method be discontinued immediately. Read a Safety Alert from the American Chemical Society about the traditional procedure for this demonstration. Solution Preparation: If you need to make ≈1.0M solutions of the metallic salts follow these instructions: Label each of the 100 mL beakers with the name of the salt that it will hold. Add the solid metallic salts to the beakers: o 12.2 grams of Barium Chloride (BaCl2*2H2O) – Note: Barium chloride is highly toxic. Do not ingest the salt or solution. o 5.5 grams of Calcium Chloride (CaCl2) o 8.5 grams of Copper Chloride (CuCl2*2H2O) o 2.1 grams of Lithium Chloride (LiCl) o 3.7 grams of Potassium Chloride (KCl) o 2.9 grams of Sodium Chloride (NaCl) o 7.9 grams of Strontium Chloride (SrCl2) Add deionized water up to the 50 mL mark. Note: if you do not have 100 mL beakers, substitute another piece of glassware and use a 50 mL graduated cylinder to add the deionized water. Use a separate wooden splint to stir each of the solutions. Distribute the remaining wooden splints between the solutions. Allow the splints to sit in the solutions overnight. Alternate options: If you cannot find wooden splints you can substitute generic cotton swabs, or longer craft swabs. Soak wooden splints or cotton swabs in deionized water for a few hours and then dip them into American Association of Chemistry Teachers |1-395 2 small samples of each solid before putting them through the flame. This demonstration can easily be used as a student lab. Demonstration Procedure: View a video of this demonstration from the American Chemical Society. The day before the demonstration: o Pour ≈50 mL of each of the solutions into labelled beakers. o Add wooden splints to each beaker and allow to soak overnight. Place the beaker or cup of water next to the Bunsen burner. Use it to hold the used splints after passing them through the flame. Light a Bunsen burner with the striker. One at a time, slowly pass the wooden splints through the burner flame. Repeat with additional splints until students have identified color. The flame will color as follows: o Barium Chloride: light green o Calcium Chloride: orange red o Copper Chloride: blue/green o Lithium Chloride: pink/fuchsia o Potassium Chloride: light lilac o Sodium Chloride: yellow flame o Strontium Chloride: red or crimson flame Students should record the color on their activity sheets and use the visible light spectrum chart to estimate the wavelength or frequency of each. Put a strontium and a copper splint into the flame at the same time and ask students to identify which metals are present. FOR THE STUDENT Lesson Flame Tests - “Rainbow Demonstration” Introduction When elements are strongly heated they produce different colored flames. These colors have a specific wavelength and frequency that are unique for each element. Bohr theorized that electrons have specific energy values, which he called energy levels. Bohr thought that the energy levels for electrons were quantized, meaning that only specific energy levels were possible around the nucleus of an atom. Electrons move between energy levels by gaining or losing a specific amounts of energy. When this occurs, we say that the electron undergoes a transition from one energy level to another. An electron in a low energy level can absorb energy and undergo a transition to a higher energy level. When that electron returns to the ground state, it loses energy by emitting a photon, which is a tiny particle that behaves like a wave and travels at the speed of light. If the photon is travelling with a wavelength and frequency within the visible light spectrum, it will have a color that can be seen. The speed of light (c), wavelength () and frequency () are related by the following equation: c= In this demonstration you will observe the color of light that is emitted when different American Association of Chemistry Teachers |1-395 3 metals are heated, estimate the wavelength or frequency of the light indicated in the data table (using a copy of the visible light spectrum chart), and identify unknown metals with your observations. You will then practice calculating wavelength and frequency from the data collected. Fill in the data table as you make your observations. Report wavelength in nanometers (nm) and frequency in terahertz (THz). Compound Name Formula Metal Color Wavelength Frequency Barium Chloride Calcium Chloride Copper II Chloride Lithium Chloride Potassium Chloride Sodium Chloride Strontium Chloride UNKNOWN #1 UNKNOWN #2 Post Lab Calculations and Questions 1. Identify the two unknown metals based on the color of light they emitted: a. Unknown 1 b. Unknown 2 2. Convert the wavelength of the following metals to meters (1nm = 1x10 -9m). Then calculate the frequency of the light emitted and convert to THz (1THz = 1x10 12 Hz): a. Barium b. Calcium c. Copper d. Lithium e. Unknown 1 3. Convert the frequency of the following metals to Hz. Then calculate the wavelength of the light emitted, converting to nm: a. Potassium b. Sodium c. Strontium d. Unknown 2 4. When a glass rod is heated, a yellow-green flame is observed around the point where it is heated. What does the yellow flame indicate might be present in the glass? 5. How do you think that metallic salts are used in fireworks? American Association of Chemistry Teachers |1-395 4 6. Two bottles containing white powders have lost their labels. How could you determine which bottle contained strontium nitrate and which contained potassium sulfate? American Association of Chemistry Teachers |1-395 5