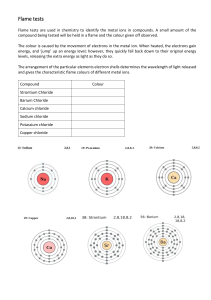
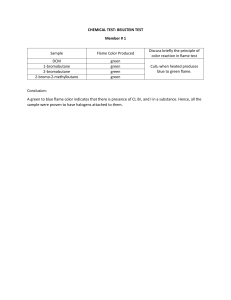
Gimnazija Bežigrad International Baccalaureate Higher Level Chemistry 2021-2022 3MM/Q EXPERIMENTAL WORK Flame test Candidate: Hana Sarajlić Supervisor: Saša Cecowski Date: 15. 9. 2021 INTRODUCTION The aim of the experiment is to observe the characteristic colours generated by certain metallic ions when burned. In practice it can be used to visually determine the possible identity of an unknown metal, or a metalloid ion found in an ionic compound. When an atom is exposed to a high temperature, one or more of its electrons gains energy and their energy levels rise. When those said atoms return to a lower energy level, the energy they give off expresses itself in the form of their characteristic colour. The higher the ion concentration in the compound, the higher the intensity of the flame colour. Variables Independent variable: Ionic compound: The compound we burn, that gives off a characteristic colour when cooled down. Dependent variable: Characteristic colours: The colours that can be seen when metalloid ions that are in the sample give off energy and gives us an idea of what the unknown compound could be. Controlled variables: The order of compounds burned. Starting with the ones that burn the fastest and ending with the ones that take a longer time to completely burn off. MATERIALS AND EQUIPMENT: Bunsen burner. Metal rod with a nichrome wire. Chemicals: Potassium Chloride, Calcium Chloride, Calcium Chloride, Copper Cloride, Barium Chloride, Lithium Cloride, Souium Chloride and an unknown sample. SAFETY NOTES: Lab coats must always be worn in the laboratory. Hands must always be disinfected or washed before leaving the laboratory. Shoes worn must be closed as to protect one’s feet. Contact between hands and face must be minimal. PROCEDURE 1. Light the Bunsen burner and adjust the air flow so the flame becomes almost colourless. 2. Clean the nichrome rod by inserting it into flame as to burn off the possible previous residue. The process is complete when there is no colour left. 3. Dip the nichrome wire in the test tube containing the compound that is to be tested. Hold the wire over the flame at a slight angle and observe the colour of the flame. 4. Clean the wire by holding it over the flame until said flame no longer has colour. 5. Repeat the procedure with each compound and clean the wire each time. 6. Repeat the steps with the unknown compound and clean the wire one final time. Picture of setup DATA COLLECTION Design your data table to accommodate both quantitative and qualitative data. Record all your raw data in tables. The tables should be numbered and have captions in which you briefly describe the contents of the tables and how you recorded the results. Titles, units and the uncertainty should be given in the headings of the tables as seen below in Table 1. Table 1: Results of the flame test Metal found in the salt Flame Color and Intensity Potassium Light-lilac, not intense. Calcium Orange-red Copper Green-blue Barium Green Lithium Red Sodium Yellow Unknown Orange-red, presumably calcium Underneath the table you can briefly describe the results. You can describe the main trends and account for any anomalous result. You don’t have to discuss the significance of the results to the aim of the investigation. DATA PROCESSING Data processing is distinct from data collection. For any calculation, first annotate for the reader the intent of your calculation. Show the equation used in symbolic form, then substitute in numbers with units. Explain any eliminated data or special treatment of the raw data made. Organize repeated calculations into a Results Table. Include any graphs in this section. The axes of the graphs have to be labelled with units and the points have to be plotted correctly. Make sure that you use the correct type of graphs. The independent variable is plotted on the x axis and the dependent variable is plotted on the y axis. CONCLUSION AND EVALUATION This section will have three distinct paragraphs. In the first paragraph, write a conclusion based on an interpretation of the gathered results. Include % error and assessment of direction and types of errors. In this section you should discuss the results you obtained in relation with your hypothesis if you stated any. In the second paragraph the procedure is evaluated. You will assess the precision and/or accuracy of your work. In the third paragraph, evaluate the limitations in the design and execution of the experiment, and suggest realistic ways to improve the experiment for future duplication of findings. Compare your results with literature values if possible. Sources: Flame test. [internet]. [cited on 15. 9. 2021]. Available on: https://askinglot.com/what-is-theaim-of-the-flame-test