Flame Test Lab: 8th Grade Science Presentation
advertisement

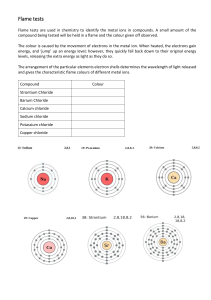
Flame Test Lab 8th Grade Science Mrs. Dainty Review Can we define the following terms? Atom Element Metal Compound Bunsen Burner Physical Characteristic Chemical Characteristic Energy Levels Light Background When certain metal compounds, or metal salts, are heated, their electrons enter an excited state. In this excited state, electrons move from lower energy levels to higher energy levels. This excited state is unstable, so the electrons quickly return to their lower energy levels. When this happens, the lose energy in the form of LIGHT. When this light is in the visible range, we can see it. (Remember Roy G. Biv!) Background Flame tests can be conducted to determine the identity of unknown elements. Certain elements give off known colors of light. These can be compared with the color of light given off by an unknown substance. Your Job: Your mission is to discover what metal elements are present in a dehydrated banana by conducting a flame test. First, you will discover the colors given off by: Calcium Chloride Copper Chloride Lithium Chloride Potassium Chloride Sodium Chloride Strontium Chloride Then, you will test a banana to see if any color change occurs in the flame. Compare these results to the known elements to draw conclusions about what’s in the banana! Limitations The flame test may not detect atoms that are present in low concentrations. The flame test cannot distinguish between ALL elements. (Some elements create the same color. Some elements don’t create a visible change in the flame color at all.) Impurities and contaminates can affect your results. Science Notebook In your notebook, write a title and date for this lab. Next, write the question we’re investigating in your own words. Your questions should make it very clear what we are trying to learn, and should identify any variables being tested. Finally, make a prediction. (I think that…because…) Demonstration Flame test demonstration: 1st time – watch and listen 2nd time – write a materials list and procedures Safety Considerations Utmost care must be taken during this lab. Absolutely no goofing around will be tolerated. Minimize movement around the room, as well as talking with your hands! (Materials Manger only one to walk around to get supplies.) Goggles, gloves, and lab coats are to be worn. Long hair must be tied back. Know locations of emergency equipments: eye wash, shower, fire blanket, fire extinguishers, etc. Voices should be kept low. In case of emergency, the FIRST thing you do is to find your teacher! Follow instructions for disposal of chemicals. Science Notebook Once you have written down the materials and procedures, develop a table in your results section. You must have your table ready before you can begin the lab. Chemicals you’ll test: Calcium Chloride Copper Chloride Lithium Chloride Potassium Chloride Sodium Chloride Strontium Chloride Unknown (Banana) Lab Day Lab Groups: Get goggles, gloves, and lab coats. Place books at tables near sinks. Bring ONLY lab notebooks to work station at counter. Go to your designated lab station. Clear away unneeded materials. Designate group roles. (If only 3, share spokesperson responsibilities.) Wait for permission to begin. Non-Lab Groups: Get goggles. Choose a place to sit near the windows. DO NOT SIT BY ANYONE WHO WILL TEMPT YOU TO GOOF OFF. My attention needs to mainly be with lab groups and I will have little patience for goofing around today! Work: Ch. 3, Lesson 4 If finished, can take turns “building an element.” (Further instructions to come.)