Covalent Bonding Review Name________________________________ Period_________Date___________________
advertisement

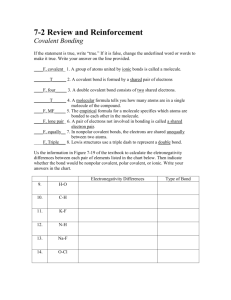
Covalent Bonding Review Name________________________________ Period_________Date___________________ Part I: True & False – If the statement is true, write “true.” If it is false, change the underlined word or words to make it true. 1. ______________________ A group of atoms united by ionic bonds is called a molecule. 2. ______________________ A covalent bond is formed by a shared pair of electrons. 3. ______________________ A double covalent bond consists of two shared electrons. 4. ______________________ A molecular formula tells you how many atoms are in a single molecule of the compound. 5. ______________________ The empirical formula for a molecule specifies which atoms are bonded to each other in the molecule. 6. ______________________ A pair of electrons not involved in bonding is called a shared electron pair. 7. ______________________ In nonpolar covalent bonds, the electrons are shared unequally between two atoms. 8. ______________________ Lewis structures use a triple dash to represent a double bond. Part II: Using your table of electronegativities, calculate the electronegativity difference between each pair of elements and predict whether the bond formed between them would be ionic, polar covalent, or nonpolar covalent. Electronegativity Difference 9. H-O 10. C-H 11. K-F 12. N-H 13. Na-F 14. O-Cl Type of Bond Part III: Write the Lewis Structures for each of the following molecules. Indicate each bond (shared pair of electrons) with a dash, and each unshared pair of electrons with two dots as directed in class. 15. NH3 18. PCl3 16. H2 19. CCl4 17. C2H4 20. C2H6 Part IV: Short answer 21. Explain the relationship between electronegativity and polarity: ______________________________ __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ 22. Compare and contrast single, double and triple covalent bonds: ______________________________ __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________