Industrial Applications of Electrolysis Objective
advertisement

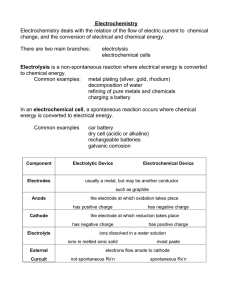
Industrial Applications of Electrolysis Objective lesson 6 chapter 13 You will be able to describe, and explain, how electrolysis is used in common production facilities. A common production method for sodium hydroxide, hydrogen gas, and chlorine gas, is the chlor-alkali cell. Here sodium chloride undergoes electrolysis as shown below. NaCl electrolysis Anode (+) Cathode (-) Net Reaction 2 NaCl(aq)+2H2O(l) ➔ H2(g)+Cl2(g)+2NaOH (aq) Down's Cell Molten (fused) NaCl is used to produce purified chlorine gas and liquid sodium metal. Anode reaction: Cathode reaction: Hall-Hèroult Process Alumina, Al2O3, is dissolved in an industrial carbonlined vat of molten cryolite, Na3AlF6 (sodium hexafluoroaluminate), called a "cell” cathode: anode: C + 2O2- ➔ CO + 4e2 Electroplating The electroplating process is a function of electrolysis whereby solid metal dissolved in solution bonds and solidifies on surfaces through the application of electric current. The object to be plated is placed at the cathode. The anode contains the metal that will be plated onto the cathode. (usually) The electrolyte contains the ion of metal that will be plated onto the cathode. Refining Metals cathode: pure metal-reduction of Cu2+ to form copper metal at the cathode. anode: impure metal-Application of a suitable voltage to the electrodes causes oxidation of copper metal at the anode. The anode dissolves and the pure metal is plated at the cathode. The electrolyte consists of an acidic solution of CuSO . 4 This strategy can be used because copper is both oxidized and reduced more readily than water. Assignment Read Section 13.3 of your textbook. Do questions 7 and 8 on page 508, and questions 17-20 on page 510