Industrial Applications of Electrolysis Objective

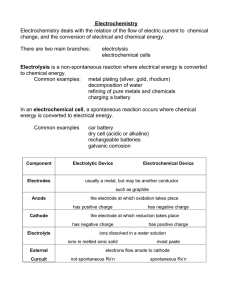
Industrial Applications of Electrolysis
lesson 6 chapter 13
Objective
You will be able to describe, and explain, how electrolysis is used in common production facilities.
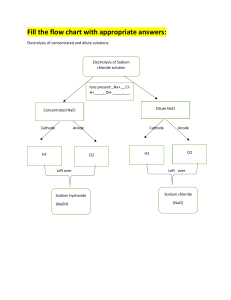
A common production method for sodium hydroxide, hydrogen gas, and chlorine gas, is the chlor-alkali cell. Here sodium chloride undergoes electrolysis as shown below.
NaCl electrolysis
Anode (+)
Cl
-
➔ 2e
-
+ Cl
2
Cathode (-)
(g)
2H
2
O(l) + 2e
-
➔ 2OH
-
(aq) + H
2
(g)
2 NaCl(aq)+2H
2
O(l) ➔ H
2
(g)+Cl
2
(g)+2NaOH (aq)
Down's Cell
Molten (fused) NaCl is used to produce purified chlorine gas and liquid sodium metal.
Anode reaction:
Cathode reaction:
Hall-Hèroult Process
Alumina, Al2O3, is dissolved in an industrial carbonlined vat of molten cryolite, Na
3
AlF
6
(sodium hexafluoroaluminate), called a "cell” cathode: Al
3+
+ 3e- ➔ Al
(s) anode: C + 2O2- ➔ CO
2
+ 4e-
Electroplating
The electroplating process is a function of electrolysis whereby solid metal dissolved in solution bonds and solidifies on surfaces through the application of electric current.
The object to be plated is placed at the cathode.
The anode contains the metal that will be plated.
(usually)
The electrolyte contains the ion of metal that will be plated.
Refining Metals
cathode: pure metal -reduction of Cu
2+
to form copper metal at the cathode.
anode: impure metal -Application of a suitable voltage to the electrodes causes oxidation of copper metal at the anode.
The anode dissolves and the pure metal is plated at the cathode.
The electrolyte consists of an acidic solution of CuSO
4
This strategy can be used because copper is both
. oxidized and reduced more readily than water.
Assignment
Read Section 13.3 of your textbook.
Do questions 7 and 8 on page 508, and questions 17-20 on page 510