Chapter 2: Measurement and Problem Solving
advertisement

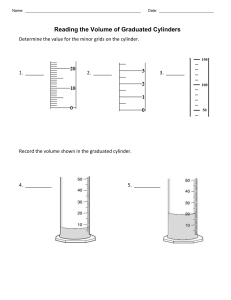
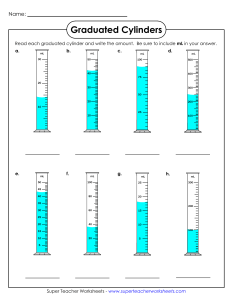
Chapter 2: Measurement and Problem Solving Practice measuring according to significant figures. What is the volume of water in the graduated cylinder in mL? How many sigfigs is this? How many L is this? Express this number in sci. notation. Always take the measurement to one decimal place past the smallest gradation (tic mark) Graduated cylinders ▪ Beakers/flasks/bottles /etc. are NOT used for measuring! Measure the volume of liquids ONLY with a graduated cylinder Rulers When using the electronic balance, record the entire number on the screen 21.75 mL 2.65 cm 3.53 cm The bathroom scale has markings at every 1 lb. Report the reading to the correct number of digits. The unit system for science measurements, based on the metric system, is called the International System of Units or SI units. The standard of length The definition of a meter, established by international agreement in 1983, is the distance that light travels in vacuum in 1/299,792,458 s. (The speed of light is 299,792,458 m/s.) The standard of mass The kilogram is defined as the mass of a block of metal kept at the International Bureau of Weights and Measures at Sèvres, France. A duplicate is kept at the National Institute of Standards and Technology near Washington, D.C. The kilogram is a measure of mass, which is different from weight. The mass of an object is a measure of the quantity of matter within it. The weight of an object is a measure of the gravitational pull on that matter. Consequently, weight depends on gravity while mass does not. The standard of time The second is defined, using an atomic clock, as the duration of 9,192,631,770 periods of the radiation emitted from a certain transition in a cesium-133 atom.