Document 17623983
advertisement

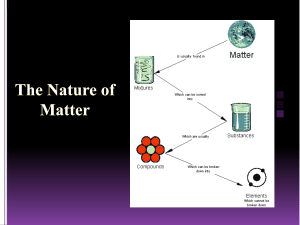
Classifying Matter Matter Are all the particles alike? YES NO Pure Substance Are the particles one kind of atom? YES Element NO Compounds or Molecules Mixture Are the particles well-mixed and mixed evenly? YES NO Homogeneous Heterogeneous Mixture Mixture Pure Element, Compound/Molecule, or Mixture? Conclusion for lab. This way of categorizing better, your way better, or both are good and why?? Pure Substances 1. Elements all particles are alike the smallest particle of an element that is still recognizable is called an atom they can’t be broken down anymore by “normal means” they are found on the periodic table currently there are about 118 elements chemical symbols are used to represent the elements • Example: C=carbon, N=nitrogen….. 2. Compounds and Molecules (over 10 million exist) two or more elements chemically combined once combined, the properties of a compound are different than the elements that make it up can be broken down into the elements that make them up example: Sugar is C6H12O6 made up of carbon, hydrogen, and oxygen looks nothing like C, H, O by themselves can be broken down when burned Mixtures 1. Heterogeneous Mixture different samples are not necessarily made up of exactly the same proportions of matter can often see different particles mixed together often can be easily separated 2. Homogeneous Mixture is the same throughout often can’t see different particles mixed together often difficult to separate examples: steel, milk, salt water, Kool Aid® History of the “Atomic Theory” video