CHEMISTRY 1411 CHAPTER 1 QUIZ.doc
CHEMISTRY 1411 CHAPTER 1 QUIZ #1
NAME:-------------------------------------------
DRIECTION: Answer any 6 of the 7 questions below.
1.
How many significant figures should you report as the multiple of 8.3801 x 2.57?
(E) none of these (A) 1 (B) 5 (C) 3
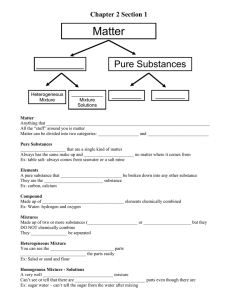
2. Which of the following statements is correct ?
(A) A compound may contain only one type of atom.
(D) 4
(B) A mixture containing two compounds must be heterogeneous.
(C) A pure substance must contain only one type of atom.
(D) A homogeneous mixture must be uniform.
(E) A heterogeneous mixture must contain at least three elements.
3. The temperature was 136.4
o F in the shade in North Texas. What is the equivalent temperature in o C?
(A) 43.8 (B) 53.2 (C) 39.8 (D) 75.8 (E) 58.0
4. How many significant figures are there in the number 0.02036101 g?
(A) 4 (B) 5 (C) 6 (D)
5. Which of these is an example of a physical property?
7 (E) 8
(A) Corrosiveness of sulfuric acid
(B) Toxicity of cyanide
(C) Flammability of gasoline
(D) Neutralization of stomach acid with an antacid
(E) Lead becomes a liquid when heated to 601
C
6. What is the specific gravity of a rock that has a mass of 77 g and has a volume of 23.5 mL? (Show your work for credit—apply all significant rules.
The Density(D) = M/V =77g/23.5ml = 3.3 g/ml. Specific Gravity(SG) = D(substance/ D(standard)
Therefore, using water (H2O) as standard, SG = 3.3g/ml/1g/ml = 3.3
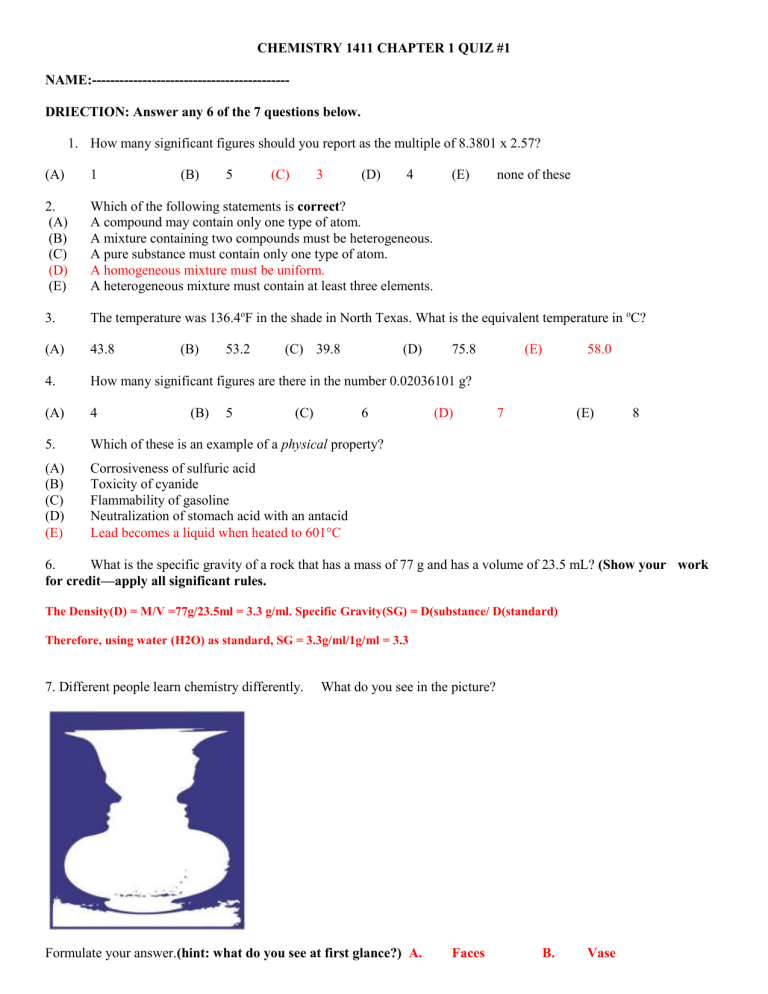
7. Different people learn chemistry differently. What do you see in the picture?
Formulate your answer.
(hint: what do you see at first glance?) A. Faces B. Vase