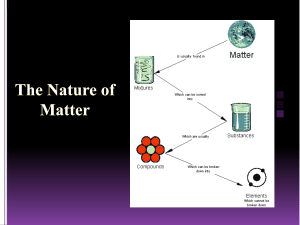
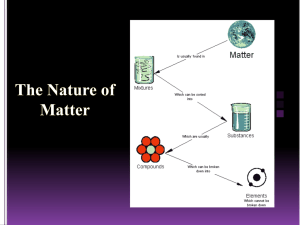
Matter Classification Flowchart: Mixtures & Pure Substances
advertisement
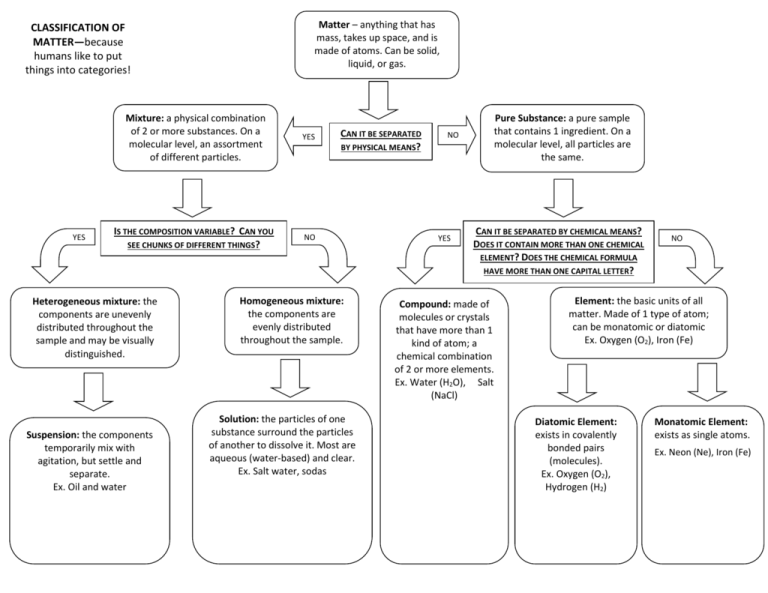
Matter – anything that has mass, takes up space, and is made of atoms. Can be solid, liquid, or gas. CLASSIFICATION OF MATTER—because humans like to put things into categories! YES Mixture: a physical combination of 2 or more substances. On a molecular level, an assortment of different particles. YES IS THE COMPOSITION VARIABLE? CAN YOU SEE CHUNKS OF DIFFERENT THINGS? NO Heterogeneous mixture: the components are unevenly distributed throughout the sample and may be visually distinguished. Suspension: the components temporarily mix with agitation, but settle and separate. Ex. Oil and water CAN IT BE SEPARATED BY PHYSICAL MEANS? Homogeneous mixture: the components are evenly distributed throughout the sample. Solution: the particles of one substance surround the particles of another to dissolve it. Most are aqueous (water-based) and clear. Ex. Salt water, sodas NO YES Pure Substance: a pure sample that contains 1 ingredient. On a molecular level, all particles are the same. CAN IT BE SEPARATED BY CHEMICAL MEANS? DOES IT CONTAIN MORE THAN ONE CHEMICAL ELEMENT? DOES THE CHEMICAL FORMULA HAVE MORE THAN ONE CAPITAL LETTER? Compound: made of molecules or crystals that have more than 1 kind of atom; a chemical combination of 2 or more elements. Ex. Water (H2O), Salt (NaCl) NO Element: the basic units of all matter. Made of 1 type of atom; can be monatomic or diatomic Ex. Oxygen (O2), Iron (Fe) Diatomic Element: exists in covalently bonded pairs (molecules). Ex. Oxygen (O2), Hydrogen (H2) Monatomic Element: exists as single atoms. Ex. Neon (Ne), Iron (Fe)