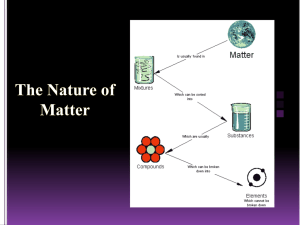
CHEMISTRY Chemistry - Deals with the study of matter and its properties, composition, reactions, structure, energy, and particles Matter - Anything that occupies space and has mass Three states of matter Solid - Has definite shape and volume Liquid - Has indefinite shape but has definite volume Gas - Has no definite shape and volume Properties of a Matter 1. Physical Properties - Can be observed without changing the composition of a substance Intensive Properties - Melting, boiling, and freezing point, density, color, physical state and thermal conductivity Extensive Properties - Mass, length, size, and volume 2. Chemical Properties - Can be observed with an accompanying change in the chemical composition Ex.: Flamability, toxicity Classification of Matter 1. Pure substance -Contains only one type of particle and has same elements all throughout 1.1 Element - Composed of only one kind of atom 1.1.1. Metal - Good conductor of heat and electricity Ex.: Na, Ca, Mg 1.1.2. Non-Metal - Poor conductor of heat and electricity Ex.: C,N,O,S 1.1.3. Semi Metal/ Metalloids - Elements which have both properties of metal and non metal 1.2. Compound - Composed of two or more elements 1.2.1. acid - donates the H+ion in an aqueous solution Ex.: Vinegar, citrus fruits 1.2.2. base - accepts the H+ion in an aqueous solution Ex.: Baking soda (NaOH), soap 1.2.3. salt - formed when acid and heat 2. Mixture - Made up of two or more substances that are not chemically bounded together 2.1. Homogenous mixture - A mixture that has a uniform solution - Can be classified as solution Ex.: Sugar and water 2.2. Heterogenous mixture - A mixture that has two or more phases - It doesn’t have a uniform composition 2.2.1. Colloid - Particle are evenly distributed throughout the mixture - Particles are larger than the particles of solution but smaller than the particles of a suspension Ex.: Mayonnaise, blood, fog, milk 2.2.2. Suspension - Particles are bigger than those particles of colloid - Solid particles are settle down Ex.: Sand and water Filtration - - It used to separate water soluble from water insoluble components It requires filtering medium (cheese cloth or filter paper) Residue - Are solid components that remains in the filtering medium Filtrate - The clear liquid collected Ex.: Silver chloride and potassium nitrate Sedimentation - Heavy suspended particles are allowed to settle at the bottom of the container - Followed by decantation Decantation -A process of seperating a solid from a liquid by pouring off the liquid after sedimentation Ex.: Sand and water Dissolution - It is another method in which a moxture of a soluble and insoluble solid substances can be seperated by using appropriate solvent Ex.: Salt and sand - The process of changing solid to gas form without passing through the liquid state Ex.: Napthalene and salt mixture Distillation - A process whereby a liquid is converted into vapor by boiling and the vapor is converted back to liquid cooling It is used if the impurities are not volatile and the liquid compound does not decompose Fractional distillation - it is used to separate two or more liquids with different boiling points Crystallization - A method to separate a soluble solid from its solution based from the different solubility of solid in water at 25 degree celcius Chromatography - A process which utilizing the strategy that lets the mixture flow over the materials at different speeds - it has two components: • The mobile phase • The stationary phase Centrifugation - aprocess of separation mixtures by applying centripetal force to a mixture using a centrifuge machine Use of magnet - It is used when separating metallic and non-metallic substances Use of Separatory Funel Sublimation - it is used to facilitate the separation of liquid to liquid mixture that did not mix - Refers to the degree to which several measurement are very close to each other Evaporation -It involves the application of heat to the solution to allow the solvent to evaporate leaving behind the solid component as a residue Rules in Counting the Significant Figure 1. Every non zero digit in a reported measurement are SIGNIFICANT 2. Left most zeros before the first non zero digit are NOT SIGNIFICANT, they only get as a placeholder to show the position of decimal pount 3. Zeros in between non zero digits are significant 4. Zeros to the right of a non zero digit and to the right of the decimal point are significant 5. Zeros at the right modt end of a measurement that lie to the left of an “understood decimal point” are not significant if they serve as placeholders. If such zeros were known measured values then they would be significant Ex.: 1. 791.22=5 2. 0.00356=3 3. 310.009=6 4. 1000=1 Rules in Operations involving significant figures For ADDITION and SUBTRACTION Least decimal place For MULTIPLICATION and DIVISION Least significant figure Precision and Accuracy Accuracy - Measurement refers to the how close the measured values is to the true value or accepted value Precise Dalton’s Atomic Theory 1. Matter is made up of extremely small indivisible particles called Atoms 2. Atoms of the same element are identical and are different from those of other elements 3. Compounds are formed when atoms of different elements combine in certain whole number ratios 4. Atoms rearrange only during a chemical reaction to form new compounds Fundamental/ Basic Laws of Matter 1. Law of Conservation of Mass - States that in a chemical reaction, matter is neither created nor destroyed, or more accurately, there is no detectable change in mass during an ordinary chemical reaction 2. Law of Definitie Proportions - States that different samples of any pure compound contain the same elements in the same proportion by mass 3. Law of Multiple Proportion - States that the mass of one element that can combine with a fixed mass of another element are in a ratio of small whole numbers Antoinne Lavoisier - Proponent of law of conservation of mass French chemist Isotopes -Same atomic number but different number of neutrons Joseph Louis Roust - Proponent of law of definite proportions - French chemist Jonh Dalton - Proponent of law of multiple proportion British scientist Subatomic Particles 1. Electron (e-) - Was discovered by J.J Thomson - Negatively charged - Plum pudding model 2. Proton (p) - Was discovered by Ernest Rutherford (gold foil experiment) and Eugene Goldstein (cathode ray tube) - Positiveley charged 3. Neutron (n) - Was discovered by James Chadwick Determining the number of p, n, e, in a nuetral atom Atomic number= no. of p= no. of e Mass no.= no. of p + no. of n No. of n= mass no. – atomic no. Quantum Numbers - Gives an information about the atomic orbital where and electron may be found 1. Principal quantum numbers (n) - It indicates the energy level or shell where an atomic orbital can be found 2. Azimuthal quantum numbers (r) - It specifies the sublevel within a particular energy level 3. Magnetic quantum numbers - It indicates the specific orbital within the sublevel where the electron is found 4. Spin quantum numbers - According to the Pauli Exclusion Principle, only a maximum two electrons can occupy an orbital and they must have opposite spins to minimize repulsion between them lower energies than those with highes energies Hund’s Rules of Maximum Multiplicity - States that for degenerate orbitals, the electrons will singly occupy each orbital and with parallel spins before they pair up Electron Configuration - Shows the distribution of electrons among the orbitals in an atom Aufbau Principle - States that electrons must first occupy the orbitals with