gas law notes 1
advertisement

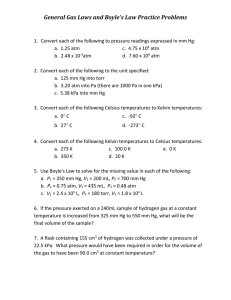
Gas Law Notes Objectives: Explain the laws of Boyle, Charles, Gay-Lussac in terms of KMT Use the above laws and the combined gas law and perform calculations. Gas Laws: Definitions: Pressure: Volume: Temperature: Memorize the combined gas law: 1. The volume of a gas is 27.5 mL at 22.0 OC and 0.974 atm. What will the volume be at 15 OC and 0.993 atm 2. A 700. mL gas sample at STP is compressed to a volume of 200. mL, and the temperature is increased to 30.0 OC. What is the new pressure of the gas in kPa? Boyle’s Law: 3. A balloon filled with helium gas has a volume of 500. mL at a pressure of 1.00 atm. The balloon is released and reaches an altitude of 6.50 km, where the pressure is 0.500 atm. Assuming that the temperature remains the same, what volume does the gas occupy at this height? 4. If some oxygen gas at 101 kPa and 25 OC is allowed to expand from 5.0 dm3 to 10.0 dm3 without changing the temperature, what pressure will the oxygen gas exert? Charles’ Law: 5. A helium filled balloon has a volume of 2.75 L at 20.0 OC. The volume of the balloon decreases to 2.46 L after it is placed outside on a cold day. What is the outside temperature? 6. A gas at 65.0 OC occupies 4.22 L. At what Celsius temperature will the volume be 3.87 L, assuming the same pressure? Gay–Lussac’s Law: 7. Before a trip from New York to Boston the pressure in an automobile tire is 1.80 atm at 20.0 °C. At the end of the trip, the pressure gauge reads 1.90 atm. What is the new Celsius temperature of the air inside the tire? (Assume tires with constant volume). 8. At 120. OC, the pressure of a sample of nitrogen is 1.07 atm. What will the pressure be at 205 OC, assuming constant volume? Avogadro’s Law: The volume of a gas is proportional to the number of moles of gas present when temperature and pressure are held constant. This example problem demonstrates how to use Avogadro's law to determine the volume of a gas when more gas is added to the system. 9. A 6.0 L sample at 25 °C and 2.00 atm of pressure contains 0.5 moles of a gas. If an additional 0.25 moles of gas at the same pressure and temperature are added, what is the final total volume of the gas? 9. What are the STP pressures? 10. What is STP in K?