Chapter 5 notes The structure and function of Macromolecules
advertisement

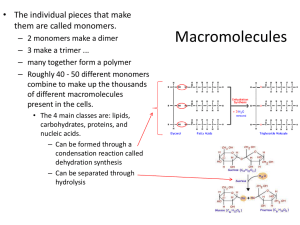
Chapter 5 notes The structure and function of Macromolecules Concept 5.1 Polymer: a long molecule consisting of similar or identical building blocks - like a train with many cars - monomers: repeating units that are the building blocks Diversity of life is based around 40 or 50 polymers Concept 5.1 Monomers are connected together by dehydration synthesis (anabolic) - covalent bond w/ the loss of H2O Polymers are broken down by hydrolysis (catabolic) - reverse rxn. of dehydration synthesis - “break with water” Concept 5.1 HO 1 2 3 H (a) 1 2 H Unlinked monomer Short polymer Dehydration removes a water molecule, forming a new bond HO HO H2O 3 4 H Longer polymer Dehydration reaction in the synthesis of a polymer Concept 5.1 HO 1 2 3 4 Hydrolysis adds a water molecule, breaking a bond HO 1 2 3 H HO H H2O H Concept 5.2 Carbohydrates: include sugars and polymers Monosaccharides (monos = single; sacchar = sugar) - molecular formula is CH2O - ex. Glucose (C6H12O6) - most sugars are rings when aqueous Concept 5.2 (a) Linear and ring forms (b) Abbreviated ring structure Concept 5.2 Disaccharide: two monosaccharides joined by a glycosidic linkage - formed by dehydration synthesis - glucose + glucose = maltose + H2O - glucose + fructose = sucrose + H2O Concept 5.2 Glucose Glucose Maltose (a) Dehydration reaction in the synthesis of maltose Glucose Fructose Sucrose (b) Dehydration reaction in the synthesis of sucrose Concept 5.2 Polysaccharides: polymers w/ few hundred to a few thousand monomers -fcn. of a polysaccharide is determined by monomers and positions of glycosidic linkages Concept 5.2 Storage polysaccharides - starch: found in plants; consists only of glucose monomers (1-4 linkage); how plants store glucose (chloroplasts) - glycogen: polymer of glucose found in animals; stored in liver and muscle cells Concept 5.2 Structural polysaccharides - cellulose: major component of plant cell walls - note: 2 ring structures of glucose (alpha (a) and beta (b)) - cellulose is composed of all b glucose - cellulose = “insoluble fiber” Concept 5.2 a Glucose (a) a and b glucose ring structures b Glucose Concept 5.2 Concept 5.2 Cell walls Cellulose microfibrils in a plant cell wall Microfibril 10 µm 0.5 µm Cellulose molecules b Glucose monomer Carbohydrates Structural polysaccharides (cntd.) - chitin: carbohydrate used by arthropods to build exoskeletons - also used to make decomposable surgical thread Concept 5.2 (a) The structure of the chitin monomer. (b) Chitin forms the exoskeleton of arthropods. (c) Chitin is used to make a strong and flexible surgical thread. Concept 5.3 Lipids: little or no affinity for water; consist mostly of hydrocarbons - 3 families: fats, phospholipids, steroids Fat: composed of 2 parts - glycerol: 3 carbon alcohol w/ hydroxyl - fatty acids: long carbon skeletons w/ carboxyl groups Concept 5.3 Fatty acid (palmitic acid) Glycerol (a) Dehydration reaction in the synthesis of a fat Concept 5.3 Fatty acids can vary in length and in the number and location of double bonds - “saturated”: no double bonds; most animal fats; solid at room temp. - “unsaturated”: has one or more double bonds which removes H atoms; plants and fish; liquid at room temp. Concept 5.3 Structural formula of a saturated fat molecule Stearic acid, a saturated fatty acid (a) Saturated fat Concept 5.3 Structural formula of an unsaturated fat molecule Oleic acid, an unsaturated fatty acid cis double bond causes (b) Unsaturated fat bending Concept 5.3 The major fcn. of fats is energy storage. - 1g of fat stores more than twice as much energy as 1g of a polysaccharide - mammals stock food reserves in adipose cells Concept 5.3 Phospholipids: similar to fats but have only 2 fatty acid tails; 3rd hydroxyl group joins to a phosphate group - show ambivalent behavior to water - head= polar (hydrophilic); tail= nonpolar (hydrophobic) - arranged in a bilayer, or double layer Hydrophobic tails Hydrophilic head Concept 5.3 Choline Phosphate Glycerol Fatty acids (a) Structural formula Hydrophilic head Hydrophobic tails (b)Space-filling model (c) Phospholipid symbol Concept 5.3 Steroids: lipids with a carbon skeleton consisting of 4 fused rings - differ in functional groups attached to rings - Cholesterol: found in animal cell membranes; precursor for other steroids Concept 5.3 Concept 5.4 Proteins: account for more than 50% of the dry weight of most cells - used for structural support, storage, transport, signaling, movement, and defense Concept 5.4 Proteins are polymers constructed from the same set of 20 amino acids - called polypeptides - consist of one or more polypeptides folded and coiled into specific conformations Concept 5.4 Amino acids are the building blocks of proteins - a carbon is bonded to an animo group, a carboxyl group, a hydrogen atom, and a variable (R) - grouped according to side chains (nonpolar, polar, acidic, basic) Concept 5.4 Concept 5.4 Concept 5.4 Amino acids are bonded together by a peptide bond - carboxyl group of one amino acid connects w/ the amino group of another (dehydration synthesis) Concept 5.4 Concept 5.4 Four levels of protein structure - primary structure: unique sequence of amino acids - even a slight change can affect a proteins conformation and ability to function - ex. Sickle-cell disease Concept 5.4 Concept 5.4 - secondary structure: coils or folds that are a result of hydrogen bonds at regular intervals - a helix: delicate coil held together by hydrogen bonding between every fourth amino acid - b pleated sheets: two or more regions lie parallel to each other Concept 5.4 Concept 5.4 - tertiary structure: irregular contortions from interactions between side chains (R groups) - hydrophobic interactions: nonpolar side chains cluster in the core, away from water - van der Waals interactions help hold them together Concept 5.4 - disulfide bridges: covalent bond between two cysteine monomers (have sulfhydryl groups) - ionic bonds and hydrogen bonds also contribute Concept 5.4 Concept 5.4 - quaternary structure: overall protein structure resulting from combining of multiple subunits The unique conformation endows each protein with a specific function Concept 5.4 Concept 5.4 Concept 5.4 The unique conformation endows each protein with a specific function - denaturation: protein unravels and losses its conformation - pH, [salt], temperature Concept 5.5 Compounds that are responsible for determining the amino acid sequence of a polypeptide. Two types of nucleic acids - deoxyribonucleic acid (DNA) - ribonucleic acid (RNA) Flow of genetic information: DNA RNA Protein Concept 5.5 DNA 1 Synthesis of mRNA in the nucleus mRNA NUCLEUS CYTOPLASM mRNA 2 Movement of mRNA into cytoplasm via nuclear pore Ribosome 3 Synthesis of protein Polypeptide Amino acids Concept 5.5 Nucleotides are the monomers (building blocks) of nucleic acids -nucleotide = nitrogenous base + pentose (5-carbon sugar) + phosphate Concept 5.5 5 end Nitrogenous bases Pyrimidines 5C 3C Nucleoside Nitrogenous base Uracil (U, in RNA) Cytosine (C) Thymine (T, in DNA) Purines Phosphate group 5C Sugar (pentose) Adenine (A) Guanine (G) (b) Nucleotide 3C 3 end Sugars (a) Polynucleotide, or nucleic acid Deoxyribose (in DNA) Ribose (in RNA) (c) Nucleoside components: sugars Concept 5.5 Two families of nitrogenous bases: - pyrimidines: single ring; cytosine (C), thymine (T), and Uracil (U) - purines: double ring; adenine (A), and guanine (G) Concept 5.5 Difference between DNA and RNA is in the sugar. DNA lacks an oxygen atom attached to its number 2 carbon Polynucleotide: nucleotides are joined by phosphodiester linkage Concept 5.5 DNA molecules have two polynucleotides that form a double helix. - Watson and Crick (1953) - A binds to T; C binds to G; forms two complementary strands