9. Atmosphere, Weather and Climate
advertisement

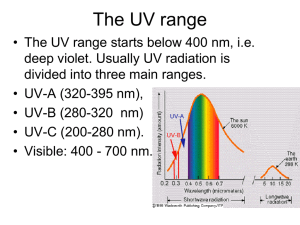
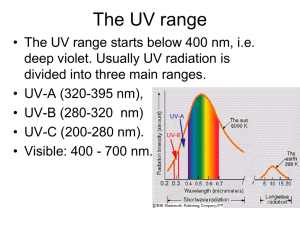
ATMOSPHERE, WEATHER AND CLIMATE The Atmosphere: In this segment we discuss the composition and structure of the atmosphere, and its influence on earth’s physical systems Earth’s atmosphere has evolved over the last 4 .6 billion years – Now, its composition and structure is relatively stable Characteristics of the Atmosphere Our atmosphere is a dynamic mixture of various gases that envelops earth and protects it from excessive and harmful solar radiation, and other onslaught, from outer space It is a thin film of air, extending for about 300 miles above Earth Held together by Earth’s gravity, it has mass, and it exerts pressure on Earth’s surface It serves as an insulator, maintaining viable temperatures on Earth It also serves as a shield, blocking out much of sun’s ultraviolet radiation, and protecting us from meteor showers and space debris Its importance becomes apparent when compared with conditions on our moon Composition of the Atmosphere Permanent or “Constant” Gases • Nitrogen (78%) • Oxygen (21%) • Argon (0.93%) These gases are called "permanent gases" because their concentration remained virtually the same for much of recent earth history. These are important for maintaining life and driving a number of processes near Earth’s surface Variable or “Greenhouse” Gases Even though they represent a tiny portion of the atmosphere as a whole (‹ 0.07%), they exert a great control over our environment • Carbon Dioxide • Methane • Ozone • Water Vapor + Particulates • The Greenhouse Effect Greenhouses keep the temperature inside warmer than outside. Sunlight (shortwave radiation) passes through the panes of glass. Materials within the greenhouse (plants, floors, walls, benches, etc.) absorb this energy. • Absorbed radiation is reradiated as heat (long-wave radiation). The glass panes block the long wave radiation, thus trapping the heat, keeping the air warm inside the greenhouse. • Carbon Dioxide (CO2) makes up only .036% of the atmosphere by volume. Carbon dioxide is essential to the photosynthesis processes of plants, in which oxygen is a byproduct • Methane (CH4) makes up far less of the atmosphere (.0002%) than carbon dioxide, but it is 20+ times more potent than CO2 as a greenhouse gas. Methane has been on the rise over the last several decades • Ozone (O3) is both beneficial and harmful to life on Earth. Much of the ozone in the atmosphere is found in the stratosphere [stratosphere] There, ozone absorbs UV light from the Sun, preventing it from reaching the surface Ozone is also found in the lowest layer of the atmosphere, the troposphere. Here ozone can act as an eye and respiratory irritant. Ozone also causes cellular damage inside the leaves of plants, disrupting the photosynthesis apparatus. The Ozone Hole Problem Without the ozone blanket, humans would be exposed to serious sunburn and potential risk of skin cancer. However, human-produced compounds such as chlorofluorocarbons (CFCs) and halides containing chlorine and bromine destroy ozone, and have disrupted the fragile stratospheric ozone layer over the Antarctic and the Arctic – the “Ozone Hole” Ozone Hole over Antarctica Sep 2009 – 9.2 Million Square Miles • Water Vapor water in gaseous state – is an extremely important gas found in the atmosphere – mostly in the lower atmosphere (troposphere). It can vary from 4% in the steamy tropics to nearly nonexistent in the cold dry regions of the Antarctic. Water vapor is a good absorber of earth's outgoing radiation and thus is considered a greenhouse gas. • Particulates play several important roles in atmospheric processes. Include dust, dirt, soot, smoke, and tiny particles of pollutants, and they are concentrated in the troposphere. Major natural sources of particulates are volcanoes, fires, wind-blown soil and sand, sea salt, and pollen. Human sources such as factories, power plants, trash incinerators, motor vehicles, and construction activity also contribute particulates to the atmosphere. Colors of the Atmosphere Due to atmospheric scattering of the wavelengths of the visible light spectrum See p. 94 in Text: The most spectacular sunrises and sunsets are a result of light being refracted from particulates in the atmosphere. Structure of the Atmosphere: Vertical Layers by Temperature Characteristics Troposphere (the Lower Atmosphere) Stratosphere (the Upper Atmosphere Starts here) The properties of the first two layers – Troposphere and Stratosphere – affect most of what we study in physical geography, especially weather & climate Together, these 2 layers extend to about 30 miles above Earth’s surface Layers above the stratopause have relatively little impact on our environment 99.9 percent of the gases (by volume) that comprise earth’s atmosphere is to be found in these two layers, and 98% of its mass lies within 16 miles above sea level Most of the water vapor and particulates in the atmosphere are concentrated in one layer – the troposphere, which is also characterized by “turbulent” air – an “ocean of air” Most of the ozone concentration – the ozone layer – is confined within the stratosphere, generally characterized by “stagnant” air Mesosphere and Beyond • In the Mesosphere, which extends to 50 miles above sea level, air temperatures begin to decrease with increasing altitude • The air of the mesosphere is thus extremely thin and air pressure very small • The Mesopause separates the mesosphere from the thermosphere above • In the Thermosphere temperature increases with increasing altitude – temperatures are generally high, but the heat content is very low due the low density of air at this level • The thermosphere extends to about 120 miles, where it starts to gradually merge into Exosphere, which in turn merges into interplanetary space – so there is no “top” of the atmosphere! Layers by Chemical Composition Layers by Function Layers by Chemical Composition Emphasis on the chemical makeup of the atmosphere led to another dichotomous layering system: Homosphere and Heterosphere. Homosphere, extending to about 50 miles above the surface, is virtually homogenous in terms of the proportions (percent by volume) of the various gases, except for a few areas of concentration of a few gases, like ozone and water vapor. Heterosphere starts from an altitude of about 50 miles and extends into the vacuum of outer space, and within it atmospheric gases are no longer evenly mixed but separated into distinct sublayers of concentration, caused by earth’s gravity in which the heavier gases are pulled down and the lighter gases drift farther outward. The nitrogen sublayer is lowermost, followed by atomic oxygen, then by helium, and then atomic hydrogen. This sphere corresponds to thermosphere and ionosphere in terms of its altitudinal limits. Layers by Functional Characteristics Another system, using protective function as the determining criterion, identified two atmospheric layers: the Ozonosphere and the Ionosphere The ozonosphere is the concentrated layer of ozone found in the stratosphere, 10 – 30 miles above the surface. Ozone (O3) absorbs ultraviolet light, and even though relatively constant through millions of years, seasonal fluctuations of ozone, especially over the Arctic and Antarctic, are common. The ionosphere, 40 – 250 miles above the surface, is not really a layer of the atmosphere, but an electrified field of ions and free electrons – it absorbs cosmic rays, gamma rays, X-rays, and shorter wavelengths of ultraviolet radiation. The spectacular display of aurora lights are generally found in this region. Effects of the Atmosphere on Insolation, and Earth’s Radiation Budget As sun’s energy passes through Earth’s Atmosphere, the actual amount of Insolation, or Incoming Solar Radiation, received at a particular location depends on many factors: • Latitude • Time of Day • Time of Year All the above are also related to the angle of sun’s rays • Atmospheric Thickness • The Transparency of the Atmosphere (which includes the amount of cloud cover, moisture/water vapor, carbon dioxide, and particles in the air) Together, all these factors determine what is called: Earth’s Radiation Budget (See diagram 3.15, p. 60) Earth’s Radiation Budget To summarize the Radiation Budget: 34% of the insolation is returned to space; 19% is retained in the atmosphere, and 47% eventually reaches the surface. The 47% received at the surface is ultimately returned to the atmosphere by various Heat Energy Transfer processes, and the Earth’s Radiation Budget is said to be in Equilibrium. However, the radiation budget is a dynamic one – alterations in one element affect the other elements – and there is growing concern that one of the elements, human activity, is causing the atmosphere to absorb more Earth-emitted energy, thus raising global temperatures. [Greenhouse Effect and Global Warming] Earth as a Greenhouse The Greenhouse Effect in the Earth context is defined as a system in which sun’s shortwave radiation enters freely and is absorbed, then reradiated as long-wave infrared radiation. The long-wave radiation is largely retained within the system, as much of it is absorbed by the greenhouse gases and reemitted in all directions, adding additional heat energy to the atmospheric and earth systems, thereby continuing the generally beneficial Greenhouse Effect. Without Greenhouse Effect, the heat would escape to outer space ← acts as Insulator Average temperature of the Earth would drop from an average of +15 degrees to -18 degrees Celsius (59˚F to – 65˚F) Life as we know it could not survive! Potential Negative Consequences How can the Greenhouse Effect be harmful to us? Rising levels of greenhouse gases evidently cause a rise in global temperatures: Global mean surface temperatures have increased by .5 - 1˚ F since the later part of the 19th century Rising temperatures have caused a decrease in snow cover and sea ice in the Northern Hemisphere Global sea level has risen by 4-8 inches over the past hundred years – Additional sea level rise and inundation of coastal regions is feared as Antarctic ice sheets and shelves, and smaller alpine glaciers melt The frequency of extreme precipitation events has increased throughout the U.S. and the world (e.g., 2010 Pakistan Floods) Watch "Global Warming 101" Courtesy of National Geographic Obvious Next Questions: How do Insolation and Heat Energy Transfer processes relate to Weather and Climate phenomena on Earth? Weather and Climate Weather: refers to the condition of atmospheric elements at a given time, and for a specific area. Climate: average of weather conditions for 30+ years (including atmospheric anomalies). Weather and Climate are of prime interest to the Physical Geographer because they affect and are interrelated with all of Earth’s environments. 5+ Basic Elements of the Atmosphere – the main ingredients of weather and climate -- Also called Elements of Weather and Climate • Solar Energy -- Insolation and Heat Energy Transfer • • • • + Temperature Pressure Wind Precipitation Air Masses (and Fronts) to be discussed later in conjunction with Weather Systems As we will see in the next set of topics, Earth’s Weather and Climate are the results of the intricate interrelationships between Earth and the Sun, and between the component spheres of our Atmosphere and our Geosphere: