ATOMIC THEORY
advertisement

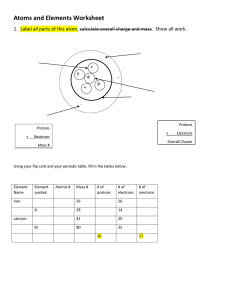
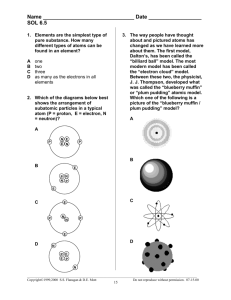
ATOMIC THEORY The Classical Atomic Theory If a substance could be divided into smaller and smaller portions of itself, eventually one should reach the level of particles that could not be divided any further. These extremely small, invisible, and indivisible particles were called atoms. Atoms of various shapes combine through interlocking patterns to form the objects of the world. Hard and compact substances (such as diamond, iron,..) contained atoms which were interlocked in a very tight pattern. In liquid substances, the atoms were thought to be held together much more loosely. Also, they thought that liquids made up of round atoms since they would pour so easily. Dalton's Atomic Theory (Modern atomic theory): • Elements are composed of small, indivisible particles called atoms. • All atoms of element such as iron are identical in mass, size, and shape, and show the same physical and chemical characteristics. • Atoms of different elements ( iron & zinc) have a different mass, size, and shape, and show different physical and chemical properties. • Atoms of two or more elements combine together to form a compound. The smallest particle that still has the properties of the compound is called a molecule. Internal Structure of the Atom Atom is made up of three subatomic particles: protons, neutrons, and electrons. PROTON: It is an elementary particle with a mass of 1.67×10-24g and has the smallest unit of positive charge. Protons will repel each other, attract particles with negative charges, and do not interact with particles that carry no charge. ELECTRON: It has the lowest mass, only 1/1836 that of a proton and has a negative charge which is equal in magnitude to that of the proton. Electrons repel each other, attract protons, and do not interact with electrically neutral particles. NEUTRON: It is a subatomic particle with a mass almost equal to that of the proton but with no electrical charge. Because of its electrically neutral nature, this particle will neither attract nor repel positively charged protons, negatively charged electrons, or other neutrons. Particle Relative mass Relative charge Proton 1 +1 Neutron 1 0 Electron 1/1836 -1 The Atomic Nucleus according to Rutherford Model (Solar system model): • Atom has central positive nucleus. The entire mass of the atom is concentrated in its nucleus and the rest of the atom was mostly empty space • The mass of electron is negligible compared to the mass of a proton or a neutron. This indicated that the protons and the neutrons are located in the nucleus, while the electrons are found in the outer regions of the atom. • The positive charge of the nucleus is determined by the number of the protons it contains. As protons and electrons have equal but opposite charges, it follows that in an electrically neutral atom the number of protons must be the same as the number of electrons. • Rutherford proposed that the electrons orbit the nucleus in the same manner that the earth and other planets orbit the sun. Therefore, Rutherford's model is often referred to as the solar-system model of the atom. Bohr Model of the Atom (electron shell model): The electron shell model assumes that the electrons orbit around the nucleus on the surfaces of imaginary spherical shells (levels). These electron shells are concentric about the nucleus in the same way as the successive layers of an onion are packed together. There are seven electron shells according to the energy level (n = 1, 2, 3, 4, 5, 6 and 7) n is known as the principal quantum number. An electron in a shell with a relatively low value of n is at a shorter distance from the nucleus than an electron in a shell with a higher value of n. Electrons in shells with low value n have a lower energy than electrons in shells with higher value of n. Each energy level of an atom could only accommodate a certain number of electrons. The maximum number of electrons is given by the following formula: Atomic Number and Nucleon Number The nucleus of an atom always contains a whole number of protons, exactly equal the number of electrons in the neutral atom. Atomic number is known as the number of protons in the nucleus of an atom. Nucleon (Mass) number is known as the sum of the numbers of protons and neutrons. Example 1: What is the atomic number of the element uranium which contains 92 electrons in each neutral atom? Solution: The number of protons must equal the number electrons in a neutral atom. Thus, the nucleus of a uranium atom contains 92 protons. The atomic number of uranium is 92. Example 2: The nucleus of an atom of fluorine contain 9 protons and 10 neutrons. What is the atomic number and the nucleon number of fluorine? Solution: Atomic number = Number of protons = 9 Nucleon number = Number of protons + Number of neutrons = 9 + 10 = 19 Isotopes Isotopes are known as atoms that have the same number of protons but a different number of neutrons in the nucleus. Thus, isotopes have the same atomic number but a different nucleon number. To distinguish between the isotopes of an element, the following symbolic representation is often used: Electron Configuration of the Elements: The arrangement of electrons in an atom is called the electron configuration. When electron fill the energy levels, it fills the lowest energy level first. Problem: Give the electron configuration for silicon (atomic number 14) according to Bohr Model of the Atom? Solution: Silicon, Si, atomic number 14 and hence 14 electrons. The first shell (K shell) can accommodate 2 electrons, and the second shell (L shell) can hold 8 electrons. That leaves 4 electrons to be accommodated in the third shell (M shell). According to the Quantum Mechanical Model of the Atom: The Quantum Mechanical Model assumes that each shell is subdivided into several number of sublevels (s, p, d and f ). There is only one s-type orbital, there are three p-type orbitals, there are five d-type orbitals, there are seven f-type orbitals. The Distribution of Electrons in each Principal Energy Level Energy Level, n Type of Atomic Orbital Number of Atomic Orbitals Maximum Number of Electrons per Sublevel Maximum Total Number of Electrons 1 2 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 1 1 3 1 3 5 1 3 5 7 2 2 6 2 6 10 2 6 10 14 2 8 3 4, 5, 6, 7 18 32 n=1 1S n= 2 2S 2P n=3 3S 3P 3d n=4 4S 4P 4d 4f n=5 5S 5P 5d 5f n= 6 6S 6P 6d 6f n= 7 7S 7P 7d 7f Name Atomic Number Electron Configuration Hydrogen 1 1s1 Helium 2 1s2 Lithium 3 1s2 2s1 Beryllium 4 1s2 2s2 Boron 5 1s2 2s22p1 Carbon 6 1s2 2s22p2 Nitrogen 7 1s2 2s22p3 Oxygen 8 1s2 2s22p4 Fluorine 9 1s2 2s22p5 Neon 10 1s2 2s22p6 Sodium 11 1s2 2s22p63s1 Magnesium 12 1s2 2s22p63s2 Aluminum 13 1s2 2s22p63s23p1 Silicon 14 1s2 2s22p63s23p2 Phosphorus 15 1s2 2s22p63s23p3 Sulfur 16 1s2 2s22p63s23p4 Chlorine 17 1s2 2s22p63s23p5 Argon 18 1s2 2s22p63s23p6 Potassium 19 1s2 2s22p63s23p64s1 Calcium 20 1s2 2s22p63s23p64s2