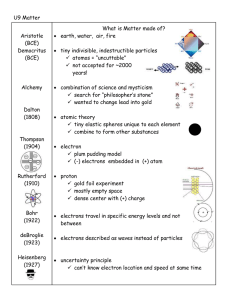
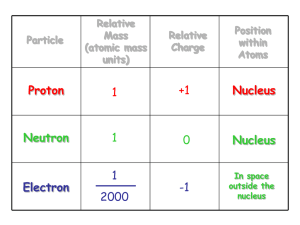
6.5
advertisement
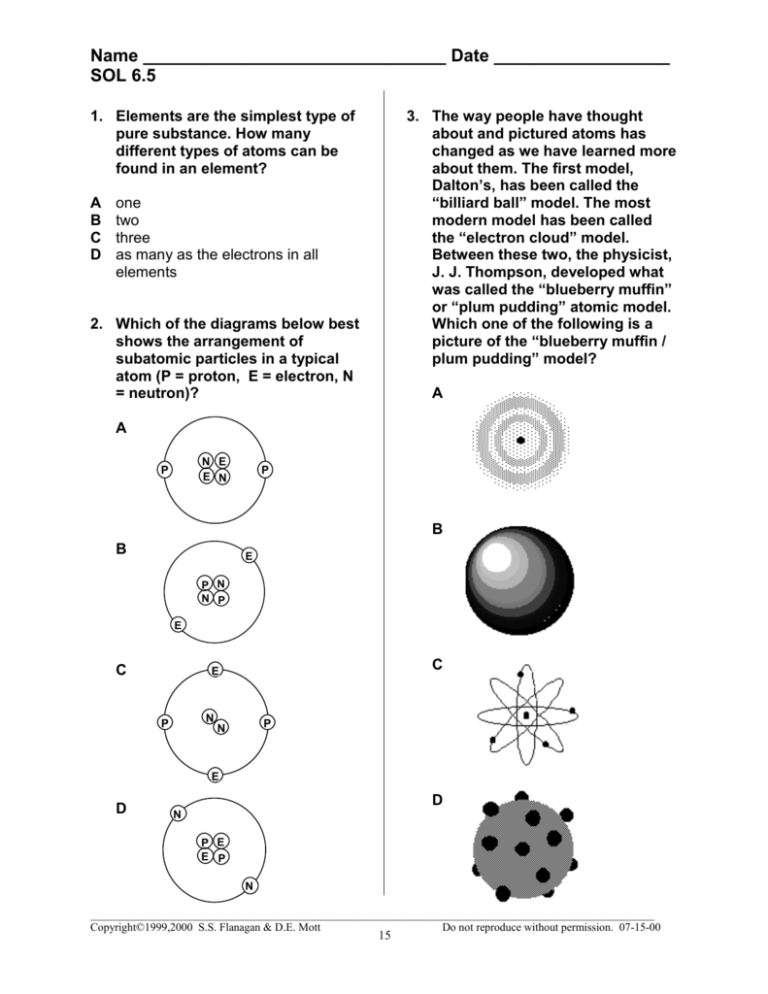
Name _______________________________ Date __________________ SOL 6.5 1. Elements are the simplest type of pure substance. How many different types of atoms can be found in an element? A B C D 3. The way people have thought about and pictured atoms has changed as we have learned more about them. The first model, Dalton’s, has been called the “billiard ball” model. The most modern model has been called the “electron cloud” model. Between these two, the physicist, J. J. Thompson, developed what was called the “blueberry muffin” or “plum pudding” atomic model. Which one of the following is a picture of the “blueberry muffin / plum pudding” model? one two three as many as the electrons in all elements 2. Which of the diagrams below best shows the arrangement of subatomic particles in a typical atom (P = proton, E = electron, N = neutron)? A A N E E N P P B B E P N N P E C C E N P P N E D D N P E E P N ____________________________________________________________________________________________________ Copyright©1999,2000 S.S. Flanagan & D.E. Mott Do not reproduce without permission. 07-15-00 15 Name _______________________________ Date __________________ SOL 6.5 4. The smallest part of a single element that has all the properties of that element is an: A B C D 8. The number of protons in the nucleus of an atom is called the: A B C D electron. atom. isotope. alloy. element number. atomic number. mass number. atomic weight. 9. A substance that contains only one kind of atom is called a(an): 5. An atomic nucleus can consist of which particles? A B C D A B C D protons and neutrons protons and electrons neutrons and electrons protons, neutrons, and electrons compound. molecule. element. nucleus. 10. The basic building block of matter is: 6. Democritus concluded that matter: A B C D A could be divided into smaller and smaller pieces forever. B could be divided until it became indivisible atoms. C consisted on protons, neutrons, and electrons. D was positively charged. 11. What is the electrical charge on an electron? A B C D 7. The small, heavy center of the atom is called a/an: A B C D compound. atom. element. mixture. neutron. proton. cortex. nucleus. + ++ -+ 12. What is the electrical charge on a proton? A B C D + ++ -+ ____________________________________________________________________________________________________ Copyright©1999,2000 S.S. Flanagan & D.E. Mott Do not reproduce without permission. 07-15-00 16