CHEM 1412 Group 1.doc
advertisement

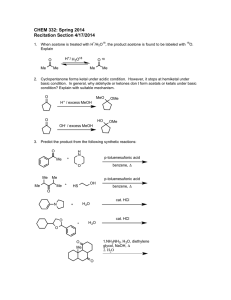
CHEM 1412 Project Group 1 About half of the hydrochloric acid produced annually in the United States (3.0 Billion pounds) is used in metal pickling. This process involves the removal of oxide layers from metal surfaces to prepare them for coating. a. write the overall and net ionic equations for the reaction between iron(III) oxide, which represents the rust layer over iron and HCl. Identify the bronsted acid and base. b. HCL is also known to remove scale(mostly CaCO3) from water pipes. This is done via a 2 stage reaction, the first stage produces the bicarbonate ion while the second stage produces carbon dioxide. write equations for each of these stages and the overall reaction. c. HCl is used to recover oil from the ground by dissolving Calcium carbonate. If the density of the solution is 1.073g/ml and the operating pH of the solution is -0.5, how many pounds of HCL would you need to order.