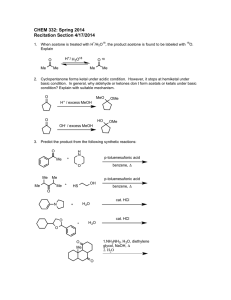
Data: One of the metals below will not react so you will put on “X”
advertisement
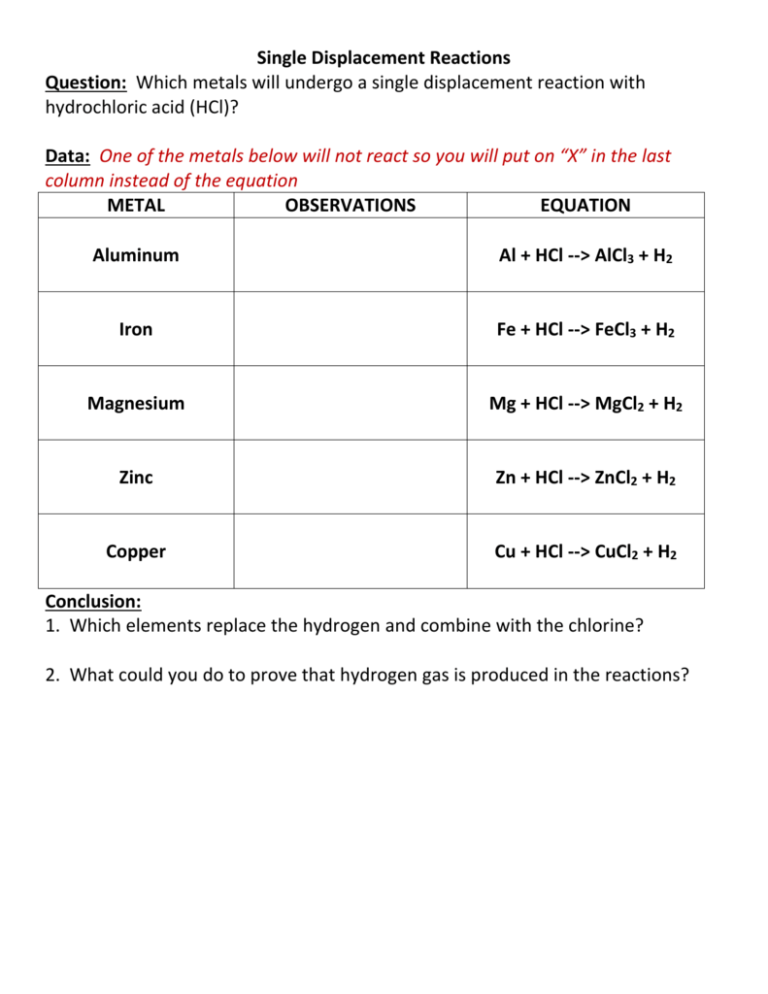
Single Displacement Reactions Question: Which metals will undergo a single displacement reaction with hydrochloric acid (HCl)? Data: One of the metals below will not react so you will put on “X” in the last column instead of the equation METAL OBSERVATIONS EQUATION Aluminum Al + HCl --> AlCl3 + H2 Iron Fe + HCl --> FeCl3 + H2 Magnesium Mg + HCl --> MgCl2 + H2 Zinc Zn + HCl --> ZnCl2 + H2 Copper Cu + HCl --> CuCl2 + H2 Conclusion: 1. Which elements replace the hydrogen and combine with the chlorine? 2. What could you do to prove that hydrogen gas is produced in the reactions?